Nuclear translocation of OsMADS25 facilitated by OsNAR2.1 in reponse to nitrate signals promotes rice root growth by targeting OsMADS27 and OsARF7
- PMID: 37353931
- PMCID: PMC10721473
- DOI: 10.1016/j.xplc.2023.100642
Nuclear translocation of OsMADS25 facilitated by OsNAR2.1 in reponse to nitrate signals promotes rice root growth by targeting OsMADS27 and OsARF7
Abstract
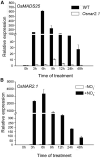
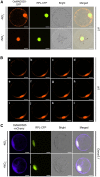
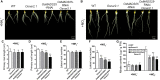
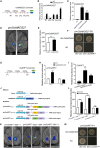
Nitrate is an important nitrogen source and signaling molecule that regulates plant growth and development. Although several components of the nitrate signaling pathway have been identified, the detailed mechanisms are still unclear. Our previous results showed that OsMADS25 can regulate root development in response to nitrate signals, but the mechanism is still unknown. Here, we try to answer two key questions: how does OsMADS25 move from the cytoplasm to the nucleus, and what are the direct target genes activated by OsMADS25 to regulate root growth after it moves to the nucleus in response to nitrate? Our results demonstrated that OsMADS25 moves from the cytoplasm to the nucleus in the presence of nitrate in an OsNAR2.1-dependent manner. Chromatin immunoprecipitation sequencing, chromatin immunoprecipitation qPCR, yeast one-hybrid, and luciferase experiments showed that OsMADS25 directly activates the expression of OsMADS27 and OsARF7, which are reported to be associated with root growth. Finally, OsMADS25-RNAi lines, the Osnar2.1 mutant, and OsMADS25-RNAi Osnar2.1 lines exhibited significantly reduced root growth compared with the wild type in response to nitrate supply, and expression of OsMADS27 and OsARF7 was significantly suppressed in these lines. Collectively, these results reveal a new mechanism by which OsMADS25 interacts with OsNAR2.1. This interaction is required for nuclear accumulation of OsMADS25, which promotes OsMADS27 and OsARF7 expression and root growth in a nitrate-dependent manner.
Keywords: OsARF7; OsMADS25; OsMADS27; OsNAR2.1; nitrate signaling; rice root.
Copyright © 2023 Zhejiang University. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
The involvement of auxin response factor OsARF7 in positively regulating root development by mediating the expression of OsCRL1 in rice (Oryza sativa L.).Plant Mol Biol. 2025 Feb 26;115(2):38. doi: 10.1007/s11103-025-01570-0. Plant Mol Biol. 2025. PMID: 40011289
-
MADS-box transcription factor OsMADS25 regulates root development through affection of nitrate accumulation in rice.PLoS One. 2015 Aug 10;10(8):e0135196. doi: 10.1371/journal.pone.0135196. eCollection 2015. PLoS One. 2015. PMID: 26258667 Free PMC article.
-
OsNAR2.2 plays a vital role in the root growth and development by promoting nitrate uptake and signaling in rice.Plant Physiol Biochem. 2020 Apr;149:159-169. doi: 10.1016/j.plaphy.2020.02.004. Epub 2020 Feb 6. Plant Physiol Biochem. 2020. PMID: 32070909
-
Nitrate transporters and mechanisms of nitrate signal transduction in Arabidopsis and rice.Physiol Plant. 2024 Jul-Aug;176(4):e14486. doi: 10.1111/ppl.14486. Physiol Plant. 2024. PMID: 39187436 Review.
-
Nitrate regulation of lateral root and root hair development in plants.J Exp Bot. 2020 Jul 25;71(15):4405-4414. doi: 10.1093/jxb/erz536. J Exp Bot. 2020. PMID: 31796961 Free PMC article. Review.
Cited by
-
Characterization of nitrate use efficiency in tea plant (Camellia sinensis) based on leaf chlorate sensitivity.Hortic Res. 2024 Dec 28;12(4):uhae354. doi: 10.1093/hr/uhae354. eCollection 2025 Apr. Hortic Res. 2024. PMID: 40046040 Free PMC article.
-
The involvement of auxin response factor OsARF7 in positively regulating root development by mediating the expression of OsCRL1 in rice (Oryza sativa L.).Plant Mol Biol. 2025 Feb 26;115(2):38. doi: 10.1007/s11103-025-01570-0. Plant Mol Biol. 2025. PMID: 40011289
-
Evolution and Function of MADS-Box Transcription Factors in Plants.Int J Mol Sci. 2024 Dec 11;25(24):13278. doi: 10.3390/ijms252413278. Int J Mol Sci. 2024. PMID: 39769043 Free PMC article. Review.
References
-
- Alvarez-Buylla E.R., García-Ponce B., Sánchez M.P., Espinosa-Soto C., García-Gómez M.L., Piñeyro-Nelson A., Garay-Arroyo A. MADS-box genes underground becoming mainstream: plant root developmental mechanisms. New Phytol. 2019;223:1143–1158. - PubMed
-
- Banda J., Bellande K., von Wangenheim D., Goh T., Guyomarc'h S., Laplaze L., Bennett M.J. Lateral root formation in Arabidopsis: a well-ordered LRexit. Trends Plant Sci. 2019;24:826–839. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

