Investigating the inhibitory property of DM hCT on hCT fibrillization via its relevant peptide fragments
- PMID: 37354016
- PMCID: PMC10360389
- DOI: 10.1002/pro.4711
Investigating the inhibitory property of DM hCT on hCT fibrillization via its relevant peptide fragments
Abstract
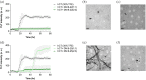
The irreversible aggregation of proteins or peptides greatly limits their bioavailability; therefore, effective inhibition using small molecules or biocompatible materials is very difficult. Human calcitonin (hCT), a hormone polypeptide with 32 residues, is secreted by the C-cells of the thyroid gland. The biological function of this hormone is to regulate calcium and phosphate concentrations in the blood via several different pathways. One of these is to inhibit the activity of osteoclasts; thus, calcitonin could be used to treat osteoporosis and Paget's disease of the bone. However, hCT is prone to aggregation in aqueous solution and forms amyloid fibrils. Salmon and eel calcitonin are currently used as clinical substitutes for hCT. In a previous study, we found that the replacement of two residues at positions 12 and 17 of hCT with amino acids that appear in the salmon sequence can greatly suppress peptide aggregation. The double mutations of hCT (DM hCT) also act as good inhibitors by disrupting wild-type hCT fibrillization, although the inhibition mechanism is not clear. More importantly, we demonstrated that DM hCT is biologically active in interacting with the calcitonin receptor. To further understand the inhibitory effect of DM hCT on hCT fibrillization, we created four relevant peptide fragments based on the DM hCT sequence. Our examination revealed that the formation of a helix of DM hCT was possibly a key component contributing to its inhibitory effect. This finding could help in the development of peptide-based inhibitors and in understanding the aggregation mechanism of hCT.
Keywords: amyloid formation; human calcitonin; mutations; osteoporosis; peptide-based inhibitors.
© 2023 The Protein Society.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

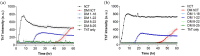




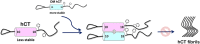
References
-
- Amodeo P, Motta A, Strazzullo G, Castiglione Morelli MA. Conformational flexibility in calcitonin: the dynamic properties of human and salmon calcitonin in solution. J Biomol NMR. 1999;13(2):161–74. - PubMed
-
- Andreotti G, Méndez BL, Amodeo P, Castiglione Morelli MA, Nakamuta H, Motta A. Structural determinants of salmon calcitonin bioactivity: the role of the leu‐based amphipathic α‐helix. J Biol Chem. 2006;281(34):24193–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

