Efficacy and Safety of Fractional CO2 Laser Combined with Halometasone Cream for Treatment of Moderate-to-Severe Chronic Hand Eczema: A Prospective, Single-Center, Parallel-Group, Open-Label Randomized Trial
- PMID: 37354295
- PMCID: PMC10366065
- DOI: 10.1007/s13555-023-00944-w
Efficacy and Safety of Fractional CO2 Laser Combined with Halometasone Cream for Treatment of Moderate-to-Severe Chronic Hand Eczema: A Prospective, Single-Center, Parallel-Group, Open-Label Randomized Trial
Abstract
Introduction: The purpose of this study was to assess the efficacy and safety of fractional CO2 laser combined with halometasone cream in patients with moderate-to-severe chronic hand eczema (CHE).
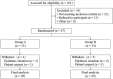
Methods: A prospective, single-center, parallel-group, open-label randomized trial including 67 patients with moderate-to-severe CHE was carried out. Patients were randomly assigned to group A (n = 33, fractional CO2 laser once every 4 weeks 1-2 times and halometasone cream twice daily for 8 weeks) or group B (n = 34, halometasone cream alone twice daily for 8 weeks). The primary endpoint was the proportion of patients achieving treatment success at week 12 in each group. Secondary endpoints included differences between groups in the change of hand eczema severity index (HECSI), patient global assessment (PaGA), dermatology life quality index (DLQI), and quality of life in hand eczema questionnaire (QOLHEQ) from baseline to week 12. Relapse rate and adverse effects were also recorded.
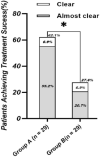
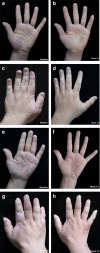
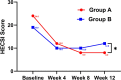
Results: A total of 29 patients in each group completed the trial. At week 12, the treatment success rate was 62.1% (18/29) in group A and 27.6% (8/29) in group B (p = 0.009). At week 12, HECSI, PaGA, DLQI, and QOLHEQ all decreased compared with baseline in both groups (p < 0.05). HECSI, DLQI, and QOLHEQ decreased more in group A than group B (p = 0.014, 0.010, and 0.014, respectively), but there was no significant difference in change of PaGA between the two groups (1.0 versus 3.0, p = 0.419). Among patients achieving treatment success, 11.1% (2/18) patients in group A and 50.0% (4/8) patients in group B relapsed at week 24 (p = 0.011). Skin pigmentation was the most common adverse effect.
Conclusions: For patients with moderate-to-severe CHE, fractional CO2 laser combined with halometasone cream is more effective than halometasone cream alone, with few adverse effects.
Trial registration number: ChiCTR2100051948.
Keywords: Efficacy; Fractional CO2 laser; Halometasone; Moderate-to-severe chronic hand eczema; Safety.
© 2023. The Author(s).
Conflict of interest statement
Gongfeng Tang, Yuan Chang, Haixuan Wu, Xuelei Liang, Yi Liu, and Fenglin Zhuo declare that they have no competing interests.
Figures
Similar articles
-
Study on different fractional CO2 laser parameters combined with halometasone in treatment of chronic eczema.Lasers Med Sci. 2025 Apr 8;40(1):183. doi: 10.1007/s10103-025-04426-7. Lasers Med Sci. 2025. PMID: 40198450 Free PMC article.
-
The pan-JAK inhibitor delgocitinib in a cream formulation demonstrates dose response in chronic hand eczema in a 16-week randomized phase IIb trial.Br J Dermatol. 2022 Jul;187(1):42-51. doi: 10.1111/bjd.21037. Epub 2022 Apr 19. Br J Dermatol. 2022. PMID: 35084738 Free PMC article. Clinical Trial.
-
Efficacy and safety of topical delgocitinib cream versus oral alitretinoin capsules in adults with severe chronic hand eczema (DELTA FORCE): a 24-week, randomised, head-to-head, phase 3 trial.Lancet. 2025 May 10;405(10490):1676-1688. doi: 10.1016/S0140-6736(25)00001-7. Epub 2025 Apr 16. Lancet. 2025. PMID: 40252681 Clinical Trial.
-
Patient-Reported Outcome Measures in Atopic Dermatitis and Chronic Hand Eczema in Adults.Patient. 2019 Oct;12(5):445-459. doi: 10.1007/s40271-019-00373-y. Patient. 2019. PMID: 31270775 Free PMC article. Review.
-
Alitretinoin for the treatment of severe chronic hand eczema.Health Technol Assess. 2010 May;14 Suppl 1:39-46. doi: 10.3310/hta14Suppl1/06. Health Technol Assess. 2010. PMID: 20507802 Review.
References
-
- Meding B. Epidemiology of hand eczema in an industrial city. Acta Derm Venereol Suppl (Stockh) 1990;153:1–43. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

