Altered Sex Differences in Hippocampal Subfield Volumes in Schizophrenia
- PMID: 37354490
- PMCID: PMC10754184
- DOI: 10.1093/schbul/sbad091
Altered Sex Differences in Hippocampal Subfield Volumes in Schizophrenia
Abstract
Background and hypothesis: The hippocampus is a heterogenous brain structure that differs between the sexes and has been implicated in the pathophysiology of psychiatric illnesses. Here, we explored sex and diagnostic group differences in hippocampal subfield volumes, in individuals with schizophrenia spectrum disorder (SZ), bipolar disorders (BD), and healthy controls (CTL).
Study design: One thousand and five hundred and twenty-one participants underwent T1-weighted magnetic resonance imaging (SZ, n = 452, mean age 30.7 ± 9.2 [SD] years, males 59.1%; BD, n = 316, 33.7 ± 11.4, 41.5%; CTL, n = 753, 34.1 ± 9.1, 55.6%). Total hippocampal, subfield, and intracranial volumes were estimated with Freesurfer (v6.0.0). Analysis of covariance and multiple regression models were fitted to examine sex-by-diagnostic (sub)group interactions in volume. In SZ and BD, separately, associations between volumes and clinical as well as cognitive measures were examined between the sexes using regression models.
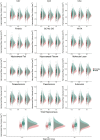
Study results: Significant sex-by-group interactions were found for the total hippocampus, dentate gyrus, molecular layer, presubiculum, fimbria, hippocampal-amygdaloid transition area, and CA4, indicating a larger volumetric deficit in male patients relative to female patients when compared with same-sex CTL. Subgroup analyses revealed that this interaction was driven by males with schizophrenia. Effect sizes were overall small (partial η < 0.02). We found no significant sex differences in the associations between hippocampal volumes and clinical or cognitive measures in SZ and BD.
Conclusions: Using a well-powered sample, our findings indicate that the pattern of morphological sex differences in hippocampal subfields is altered in individuals with schizophrenia relative to CTL, due to higher volumetric deficits in males.
Keywords: bipolar disorders; hippocampus; neuroimaging; schizophrenia; sex differences.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Maryland Psychiatric Research Center.
Conflict of interest statement
For work unrelated to the content of this manuscript, OAA and IA received speaker's honorarium from Lundbeck. OAA also received speaker's honorarium from Sunovion and Janssen, and works as a consultant for Cortechs.ai. The other authors report no biomedical financial interests or potential conflicts of interest.
Figures

References
-
- Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK.. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013;25(11):1039–1061. - PubMed
-
- Duarte-Guterman P, Yagi S, Chow C, Galea LA.. Hippocampal learning, memory, and neurogenesis: effects of sex and estrogens across the lifespan in adults. Horm Behav. 2015;74:37–52. - PubMed
-
- Bliss TV, Collingridge GL.. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. - PubMed

