Blood neurofilament light levels predict non-relapsing progression following anti-CD20 therapy in relapsing and primary progressive multiple sclerosis: findings from the ocrelizumab randomised, double-blind phase 3 clinical trials
- PMID: 37354600
- PMCID: PMC10320523
- DOI: 10.1016/j.ebiom.2023.104662
Blood neurofilament light levels predict non-relapsing progression following anti-CD20 therapy in relapsing and primary progressive multiple sclerosis: findings from the ocrelizumab randomised, double-blind phase 3 clinical trials
Abstract
Background: Neurofilament light chain (NfL), a neuronal cytoskeletal protein that is released upon neuroaxonal injury, is associated with multiple sclerosis (MS) relapsing activity and has demonstrated some prognostic ability for future relapse-related disease progression, yet its value in assessing non-relapsing disease progression remains unclear.
Methods: We examined baseline and longitudinal blood NfL levels in 1421 persons with relapsing MS (RMS) and 596 persons with primary progressive MS (PPMS) from the pivotal ocrelizumab MS trials. NfL treatment-response and risk for disease worsening (including disability progression into the open-label extension period and slowly expanding lesions [SELs] on brain MRI) at baseline and following treatment with ocrelizumab were evaluated using time-to-event analysis and linear regression models.
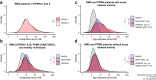
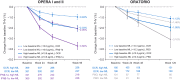
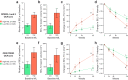
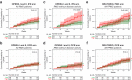
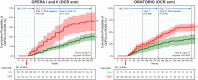
Findings: In persons from the RMS control arms without acute disease activity and in the entire PPMS control arm, higher baseline NfL was prognostic for greater whole brain and thalamic atrophy, greater volume expansion of SELs, and clinical progression. Ocrelizumab reduced NfL levels vs. controls in persons with RMS and those with PPMS, and abrogated the prognostic value of baseline NfL on disability progression. Following effective suppression of relapse activity by ocrelizumab, NfL levels at weeks 24 and 48 were significantly associated with long-term risk for disability progression, including up to 9 years of observation in RMS and PPMS.
Interpretation: Highly elevated NfL from acute MS disease activity may mask a more subtle NfL abnormality that reflects underlying non-relapsing progressive biology. Ocrelizumab significantly reduced NfL levels, consistent with its effects on acute disease activity and disability progression. Persistently elevated NfL levels, observed in a subgroup of persons under ocrelizumab treatment, demonstrate potential clinical utility as a predictive biomarker of increased risk for clinical progression. Suppression of relapsing biology with high-efficacy immunotherapy provides a window into the relationship between NfL levels and future non-relapsing progression.
Funding: F. Hoffmann-La Roche Ltd.
Keywords: Biomarker; Disease progression; Multiple sclerosis; NfL; Ocrelizumab.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests AB-O has received consulting fees from Gossamer, Janssen/Actelion, Atara Biotherapeutics, Biogen, BMS/Celgene/Receptos, F. Hoffmann-La Roche Ltd., Genentech, Inc., MAPI, MedImmune, Merck/EMD Serono, Novartis, Sanofi Genzyme, and GSK; has performed contracted research for Genentech, Inc., Novartis, and Biogen; and receives a salary from the University of Pennsylvania Perelman School of Medicine. G-AT, UB, LG, FM, AS, and HK are employees and shareholders of F. Hoffmann-La Roche Ltd. CH, SF, RH, XJ, and AH are employees of Genentech, Inc., and shareholders of F. Hoffmann-La Roche Ltd. CB has received consulting fees as a contractor for F. Hoffmann-La Roche Ltd. AHC has, in the past year, received fees or honoraria for consulting from Biogen, Celgene, EMD Serono/Merck, F. Hoffmann-La Roche Ltd., Genentech, Inc., Greenwich Biosciences, Janssen Pharmaceuticals, and Novartis. SLH serves on the SAB of Accure, Annexon, and Alector and on the BOD of Neurona, has consulted for NGM Bio, and has received travel reimbursement and writing assistance from F. Hoffmann-La Roche Ltd. and Novartis for CD20-related meetings and presentations. LK’s institution (University Hospital Basel) has received funds in the past 3 years that were exclusively used for research support at the Department, for steering committee and advisory board participation, consultancy services, and participation in educational activities from the following organisations: Actelion, AurigaVision AG, Bayer AG, BMS, Celgene, df-mp Molnia & Pohlman, Eli Lilly, EMD Serono, Genentech, GSK, Janssen LLC, Janssen Pharmaceuticals, Japan Tobacco Inc., Merck, MH Consulting, Merck Healthcare KGaA, Minoryx Therapeutics S.L., Novartis, Novartis Biociências S.A., Österreichische Gesellschaft für Neurologie, Roche, Sanofi, Santhera Pharmaceuticals, Senda Biosciences Inc., Shionogi BV, TG Therapeutics, and Wellmera AG; has served a leadership or fiduciary role for Foundation Clinical Neuroimmunology and Neuroscience Basel, MAGNIMS Steering Committee, European Charcot Foundation, and Neurostatus-UHB AG; the Research of the MS Center in Basel has been supported by grants from Novartis, Innosuisse, and Roche. JK received speaker fees, research support, and travel support and/or served on advisory boards for ECTRIMS, Swiss MS Society, Swiss National Research Foundation (grant no. 320030_189140/1), University of Basel, Bayer, Biogen, Celgene, Genzyme, Merck, Novartis, Roche, Sanofi, and Teva. DL is Chief Medical Officer of GeNeuro and has received travel reimbursement and personal compensation for consulting and speaking from Quanterix, Orion, Novartis, Roche, and Sanofi; has received consulting fees for GeNeuro SA as CMO, Orion, Novartis, Roche, and Sanofi; has received travel support from GeNeuro SA and Sanofi; and holds stock in GeNeuro SA.
Figures

References
-
- Kappos L., Wolinsky J.S., Giovannoni G., et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132–1140. doi: 10.1001/jamaneurol.2020.1568. - DOI - PMC - PubMed
-
- Genentech Ocrevus (Ocrelizumab) [Full prescribing information] https://www.gene.com/download/pdf/ocrevus_prescribing.pdf
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

