Performance of commercial SARS-CoV-2 wild-type and Omicron BA.1 antibody assays compared with pseudovirus neutralization tests
- PMID: 37354690
- PMCID: PMC10251723
- DOI: 10.1016/j.jcv.2023.105518
Performance of commercial SARS-CoV-2 wild-type and Omicron BA.1 antibody assays compared with pseudovirus neutralization tests
Abstract
Background: Commercially available ELISA-based antibody tests are used to approximate vaccination success against SARS-CoV-2 in at-risk patients, but it is unclear whether they correlate with neutralization of the Omicron variant.
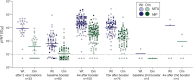
Methods: 269 serum samples of a cohort of 44 non-immunosuppressed participants and 65 MTX-treated rheumatic patients taken before and after COVID-19 booster vaccinations were measured using COVID-19 antibody testing systems with wild-type and Omicron BA.1 antigens developed by three different manufacturers (surrogate virus neutralization test cPass, and binding antibody tests QuantiVac and SeraSpot), as well as with a pseudovirus neutralization test (pVNT). The pVNT was considered the gold standard for determining the presence and level of anti-SARS-CoV-2 antibodies.
Results: All three wild-type ELISAs showed excellent test performance compared with wild-type neutralization in pVNT. However, out of 56 samples without Omicron BA.1 neutralization in pVNT, 71.4% showed positive results in at least one and 28.6% in all three wild-type ELISAs at the manufacturer-defined cut-offs. Omicron ELISAs showed either decreased specificity (57.1% and 55.4% for binding ELISAs) or sensitivity (51.2% in cPass) compared to Omicron neutralization in pVNT. The proportion of any false positive results among all samples decreased from 26.5% before to 3.2% after booster vaccination, however binding antibody test specificities remained below 70%.
Conclusions: We found a poorer test performance of new Omicron antibody test systems compared to wild-type tests in detecting neutralizing antibodies against the corresponding SARS-CoV-2 variants. Decisions for booster vaccination or passive immunization of at-risk patients should not be based solely on antibody test results.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest Euroimmun provided test kits for antibody testing free of charge as part of a research agreement. Otherwise, the authors declare that there are no conflicts of interest.
Figures

References
-
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021:1–7. doi: 10.1038/s41591-021-01377-8. - DOI - PubMed
-
- Dolscheid-Pommerich R., Bartok E., Renn M., Kümmerer B.M., Schulte B., Schmithausen R.M., Stoffel-Wagner B., Streeck H., Saschenbrecker S., Steinhagen K., Hartmann G. Correlation between a quantitative anti-SARS-CoV-2 IgG ELISA and neutralization activity. J. Med. Virol. 2022;94:388–392. doi: 10.1002/jmv.27287. - DOI - PMC - PubMed
-
- Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., Dold C., Fuskova M., Gilbert S.C., Hirsch I., Humphries H.E., Jepson B., Kelly E.J., Plested E., Shoemaker K., Thomas K.M., Vekemans J., Villafana T.L., Lambe T., Pollard A.J., Voysey M. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

