Immune response after two doses of the BNT162b2 COVID-19 vaccine and risk of SARS-CoV-2 breakthrough infection in Tyrol, Austria: an open-label, observational phase 4 trial
- PMID: 37354911
- PMCID: PMC10284585
- DOI: 10.1016/S2666-5247(23)00107-6
Immune response after two doses of the BNT162b2 COVID-19 vaccine and risk of SARS-CoV-2 breakthrough infection in Tyrol, Austria: an open-label, observational phase 4 trial
Abstract
Background: Correlates of protection could help to assess the extent to which a person is protected from SARS-CoV-2 infection after vaccination (so-called breakthrough infection). We aimed to clarify associations of antibody and T-cell responses after vaccination against COVID-19 with risk of a SARS-CoV-2 breakthrough infection and whether measurement of these responses enhances risk prediction.
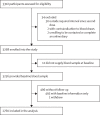
Methods: We did an open-label, phase 4 trial in two community centres in the Schwaz district of the Federal State of Tyrol, Austria, before the emergence of the omicron (B.1.1.529) variant of SARS-CoV-2. We included individuals (aged ≥16 years) a mean of 35 days (range 27-43) after they had received a second dose of the BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine. We quantified associations between immunological parameters and breakthrough infection and assessed whether information on these parameters improves risk discrimination. The study is registered with the European Union Drug Regulating Authorities Clinical Trials Database, 2021-002030-16.
Findings: 2760 individuals (1682 [60·9%] female, 1078 [39·1%] male, mean age 47·4 years [SD 14·5]) were enrolled into this study between May 15 and May 21, 2021, 712 (25·8%) of whom had a previous SARS-CoV-2 infection. Over a median follow-up of 5·9 months, 68 (2·5%) participants had a breakthrough infection. In models adjusted for age, sex, and previous infection, hazard ratios for breakthrough infection for having twice the immunological parameter level at baseline were 0·72 (95% CI 0·60-0·86) for anti-spike IgG, 0·80 (0·70-0·92) for neutralising antibodies in a surrogate virus neutralisation assay, 0·84 (0·58-1·21) for T-cell response after stimulation with a CD4 peptide pool, and 0·77 (0·54-1·08) for T-cell response after stimulation with a combined CD4 and CD8 peptide pool. For neutralising antibodies measured in a nested case-control sample using a pseudotyped virus neutralisation assay, the corresponding odds ratio was 0·78 (0·62-1·00). Among participants with previous infection, the corresponding hazard ratio was 0·73 (0·61-0·88) for anti-nucleocapsid Ig. Addition of anti-spike IgG information to a model containing information on age and sex improved the C-index by 0·085 (0·027-0·143).
Interpretation: In contrast to T-cell response, higher levels of binding and neutralising antibodies were associated with a reduced risk of breakthrough SARS-CoV-2 infection. The assessment of anti-spike IgG enhances the prediction of incident breakthrough SARS-CoV-2 infection and could therefore be a suitable correlate of protection in practice. Our phase 4 trial measured both humoral and cellular immunity and had a 6-month follow-up period; however, the longer-term protection against emerging variants of SARS-CoV-2 remains unclear.
Funding: None.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests DB holds stocks of Pfizer. The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays and Newcastle disease virus-based COVID-19 vaccines that list FK as a co-inventor. FK has received support from the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases Collaborative Influenza Vaccine Innovation Centers (contract 75N93019C00051), NIH Centers of Excellence for Influenza Research and Response (75N93021C00014), the JPB Foundation and the Open Philanthropy Project (research grant 2020-215611, 5384), and the NIH National Cancer Institute (contract 75N91019D00024, task order 75N91020F00003). FK's laboratory is also collaborating with Pfizer on animal models of SARS-CoV-2. FK has received royalties or licences from Avimex and Kantaro. The Icahn School of Medicine at Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2. FK has consulted for Pfizer (before 2020), and is currently consulting for Pfizer, Seqirus, Third Rock Ventures, and Avimex. FK has received payment or honoraria for academic lectures over the past 2 years. All other authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

