Targeted degradation of ⍺-synuclein aggregates in Parkinson's disease using the AUTOTAC technology
- PMID: 37355598
- PMCID: PMC10290391
- DOI: 10.1186/s13024-023-00630-7
Targeted degradation of ⍺-synuclein aggregates in Parkinson's disease using the AUTOTAC technology
Erratum in
-
Correction: Targeted degradation of ⍺-synuclein aggregates in Parkinson's disease using the AUTOTAC technology.Mol Neurodegener. 2023 Sep 29;18(1):69. doi: 10.1186/s13024-023-00661-0. Mol Neurodegener. 2023. PMID: 37775804 Free PMC article. No abstract available.
Abstract
Background: There are currently no disease-modifying therapeutics for Parkinson's disease (PD). Although extensive efforts were undertaken to develop therapeutic approaches to delay the symptoms of PD, untreated α-synuclein (α-syn) aggregates cause cellular toxicity and stimulate further disease progression. PROTAC (Proteolysis-Targeting Chimera) has drawn attention as a therapeutic modality to target α-syn. However, no PROTACs have yet shown to selectively degrade α-syn aggregates mainly owing to the limited capacity of the proteasome to degrade aggregates, necessitating the development of novel approaches to fundamentally eliminate α-syn aggregates.
Methods: We employed AUTOTAC (Autophagy-Targeting Chimera), a macroautophagy-based targeted protein degradation (TPD) platform developed in our earlier studies. A series of AUTOTAC chemicals was synthesized as chimeras that bind both α-syn aggregates and p62/SQSTM1/Sequestosome-1, an autophagic receptor. The efficacy of Autotacs was evaluated to target α-syn aggregates to phagophores and subsequently lysosomes for hydrolysis via p62-dependent macroautophagy. The target engagement was monitored by oligomerization and localization of p62 and autophagic markers. The therapeutic efficacy to rescue PD symptoms was characterized in cultured cells and mice. The PK/PD (pharmacokinetics/pharmacodynamics) profiles were investigated to develop an oral drug for PD.
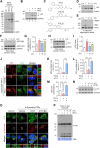
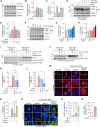
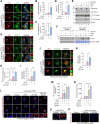
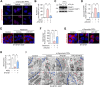
Results: ATC161 induced selective degradation of α-syn aggregates at DC50 of ~ 100 nM. No apparent degradation was observed with monomeric α-syn. ATC161 mediated the targeting of α-syn aggregates to p62 by binding the ZZ domain and accelerating p62 self-polymerization. These p62-cargo complexes were delivered to autophagic membranes for lysosomal degradation. In PD cellular models, ATC161 exhibited therapeutic efficacy to reduce cell-to-cell transmission of α-syn and to rescue cells from the damages in DNA and mitochondria. In PD mice established by injecting α-syn preformed fibrils (PFFs) into brain striata via stereotaxic surgery, oral administration of ATC161 at 10 mg/kg induced the degradation of α-syn aggregates and reduced their propagation. ATC161 also mitigated the associated glial inflammatory response and improved muscle strength and locomotive activity.
Conclusion: AUTOTAC provides a platform to develop drugs for PD. ATC161, an oral drug with excellent PK/PD profiles, induces selective degradation of α-syn aggregates in vitro and in vivo. We suggest that ATC161 is a disease-modifying drug that degrades the pathogenic cause of PD.
Keywords: Lysosome; Macroautophagy; Targeted protein degradation; The N-degron pathway; The autophagy-lysosome system; p62/SQSTM1/Sequestosome-1.
© 2023. The Author(s).
Conflict of interest statement
Seoul National University and AUTOTAC Bio Inc. have filed patent applications based on the results of this study. The remaining authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

