Analysis of the characteristics and the degree of pragmatism exhibited by pragmatic-labelled trials of antineoplastic treatments
- PMID: 37355603
- PMCID: PMC10290324
- DOI: 10.1186/s12874-023-01975-9
Analysis of the characteristics and the degree of pragmatism exhibited by pragmatic-labelled trials of antineoplastic treatments
Abstract
Background: Pragmatic clinical trials (PCTs) are designed to reflect how an investigational treatment would be applied in clinical practice. As such, unlike their explanatory counterparts, they measure therapeutic effectiveness and are capable of generating high-quality real-world evidence. However, the conduct of PCTs remains extremely rare. The scarcity of such studies has contributed to the emergence of the efficacy-effectiveness gap and has led to calls for launching more of them, including in the field of oncology. This analysis aimed to identify self-labelled pragmatic trials of antineoplastic interventions and to evaluate whether their use of this label was justified.
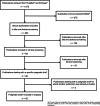
Methods: We searched PubMed® and Embase® for publications corresponding with studies that investigated antitumor therapies and that were tagged as pragmatic in their titles, abstracts and/or index terms. Subsequently, we consulted all available source documents for the included trials and extracted relevant information from them. The data collected were then used to appraise the degree of pragmatism displayed by the PCTs with the help of the validated PRECIS-2 tool.
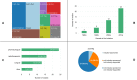
Results: The literature search returned 803 unique records, of which 46 were retained upon conclusion of the screening process. This ultimately resulted in the identification of 42 distinct trials that carried the 'pragmatic' label. These studies examined eight different categories of neoplasms and were mostly randomized, open-label, multicentric, single-country trials sponsored by non-commercial parties. On a scale of one (very explanatory) to five (very pragmatic), the median PCT had a PRECIS-2 score per domain of 3.13 (interquartile range: 2.57-3.53). The most and least pragmatic studies in the sample had a score of 4.44 and 1.57, respectively. Only a minority of trials were described in sufficient detail to allow them to be graded across all domains of the PRECIS-2 instrument. Many of the studies examined also had features that arguably precluded them from being pragmatic altogether, such as being monocentric or placebo-controlled in nature.
Conclusion: PCTs of antineoplastic treatments are generally no more pragmatic than they are explanatory.
Keywords: Cancer; Efficacy-effectiveness gap; Explanatory trials; Neoplasms; Oncology; PRECIS-2; Pragmatic trials; Randomized controlled trials; Real-world evidence; Treatments.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Real-world evidence: How pragmatic are randomized controlled trials labeled as pragmatic?BMC Med. 2018 Apr 3;16(1):49. doi: 10.1186/s12916-018-1038-2. BMC Med. 2018. PMID: 29615035 Free PMC article.
-
The PRECIS-2 tool seems not to be useful to discriminate the degree of pragmatism of medicine masked trials from that of open-label trials.Eur J Clin Pharmacol. 2021 Apr;77(4):539-546. doi: 10.1007/s00228-020-03030-8. Epub 2020 Oct 26. Eur J Clin Pharmacol. 2021. PMID: 33106910
-
Lack of pragmatic attitude of self-labelled pragmatic trials on manual therapy: a methodological review.BMC Med Res Methodol. 2024 Nov 11;24(1):273. doi: 10.1186/s12874-024-02393-1. BMC Med Res Methodol. 2024. PMID: 39528934 Free PMC article. Review.
-
Applying the PRECIS-2 tool for self-declared 'pragmatic' acupuncture trials: protocol for a systematic review.BMJ Open. 2022 Apr 12;12(4):e052861. doi: 10.1136/bmjopen-2021-052861. BMJ Open. 2022. PMID: 35414545 Free PMC article.
-
PRECIS-2 analysis of pragmatic acupuncture trials: a systematic review.BMC Complement Med Ther. 2024 May 3;24(1):181. doi: 10.1186/s12906-024-04473-7. BMC Complement Med Ther. 2024. PMID: 38702632 Free PMC article.
Cited by
-
Utilisation of radiotherapy in lung cancer: A scoping narrative literature review with a focus on the introduction of evidence-based therapeutic approaches in Europe.Clin Transl Radiat Oncol. 2023 Dec 18;45:100717. doi: 10.1016/j.ctro.2023.100717. eCollection 2024 Mar. Clin Transl Radiat Oncol. 2023. PMID: 38226026 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

