Pharmacokinetics of ivermectin metabolites and their activity against Anopheles stephensi mosquitoes
- PMID: 37355605
- PMCID: PMC10290335
- DOI: 10.1186/s12936-023-04624-0
Pharmacokinetics of ivermectin metabolites and their activity against Anopheles stephensi mosquitoes
Abstract
Background: Ivermectin (22,23-dihydroavermectin B1a: H2B1a) is an endectocide used to treat worm infections and ectoparasites including lice and scabies mites. Furthermore, survival of malaria transmitting Anopheles mosquitoes is strongly decreased after feeding on humans recently treated with ivermectin. Currently, mass drug administration of ivermectin is under investigation as a potential novel malaria vector control tool to reduce Plasmodium transmission by mosquitoes. A "post-ivermectin effect" has also been reported, in which the survival of mosquitoes remains reduced even after ivermectin is no longer detectable in blood meals. In the present study, existing material from human clinical trials was analysed to understand the pharmacokinetics of ivermectin metabolites and feeding experiments were performed in Anopheles stephensi mosquitoes to assess whether ivermectin metabolites contribute to the mosquitocidal action of ivermectin and whether they may be responsible for the post-ivermectin effect.
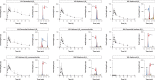
Methods: Ivermectin was incubated in the presence of recombinant human cytochrome P450 3A4/5 (CYP 3A4/5) to produce ivermectin metabolites. In total, nine metabolites were purified by semi-preparative high-pressure liquid chromatography. The pharmacokinetics of the metabolites were assessed over three days in twelve healthy volunteers who received a single oral dose of 12 mg ivermectin. Blank whole blood was spiked with the isolated metabolites at levels matching the maximal blood concentration (Cmax) observed in pharmacokinetics study samples. These samples were fed to An. stephensi mosquitoes, and their survival and vitality was recorded daily over 3 days.
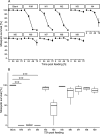
Results: Human CYP3A4 metabolised ivermectin more rapidly than CYP3A5. Ivermectin metabolites M1-M8 were predominantly formed by CYP3A4, whereas metabolite M9 (hydroxy-H2B1a) was mainly produced by CYP3A5. Both desmethyl-H2B1a (M1) and hydroxy-H2B1a (M2) killed all mosquitoes within three days post-feeding, while administration of desmethyl, hydroxy-H2B1a (M4) reduced survival to 35% over an observation period of 3 days. Ivermectin metabolites that underwent deglycosylation or hydroxylation at spiroketal moiety were not active against An. stephensi at Cmax levels. Interestingly, half-lives of M1 (54.2 ± 4.7 h) and M4 (57.5 ± 13.2 h) were considerably longer than that of the parent compound ivermectin (38.9 ± 20.8 h).
Conclusion: In conclusion, the ivermectin metabolites M1 and M2 contribute to the activity of ivermectin against An. stephensi mosquitoes and could be responsible for the "post-ivermectin effect".
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Differential susceptibilities of Anopheles albimanus and Anopheles stephensi mosquitoes to ivermectin.Malar J. 2018 Apr 3;17(1):148. doi: 10.1186/s12936-018-2296-3. Malar J. 2018. PMID: 29615055 Free PMC article.
-
Ivermectin susceptibility, sporontocidal effect, and inhibition of time to re-feed in the Amazonian malaria vector Anopheles darlingi.Malar J. 2017 Nov 21;16(1):474. doi: 10.1186/s12936-017-2125-0. Malar J. 2017. PMID: 29162101 Free PMC article.
-
Ivermectin metabolites reduce Anopheles survival.Sci Rep. 2023 May 19;13(1):8131. doi: 10.1038/s41598-023-34719-2. Sci Rep. 2023. PMID: 37208382 Free PMC article.
-
Ivermectin to reduce malaria transmission III. Considerations regarding regulatory and policy pathways.Malar J. 2017 Apr 24;16(1):162. doi: 10.1186/s12936-017-1803-2. Malar J. 2017. PMID: 28434407 Free PMC article. Review.
-
A Roadmap for the Development of Ivermectin as a Complementary Malaria Vector Control Tool.Am J Trop Med Hyg. 2020 Feb;102(2s):3-24. doi: 10.4269/ajtmh.19-0620. Am J Trop Med Hyg. 2020. PMID: 31971144 Free PMC article.
Cited by
-
Ivermectin: A Multifaceted Drug With a Potential Beyond Anti-parasitic Therapy.Cureus. 2024 Mar 12;16(3):e56025. doi: 10.7759/cureus.56025. eCollection 2024 Mar. Cureus. 2024. PMID: 38606261 Free PMC article. Review.
-
Need for a paradigm shift in soil-transmitted helminthiasis control: Targeting the right people, in the right place, and with the right drug(s).PLoS Negl Trop Dis. 2024 Oct 21;18(10):e0012521. doi: 10.1371/journal.pntd.0012521. eCollection 2024 Oct. PLoS Negl Trop Dis. 2024. PMID: 39432840 Free PMC article. No abstract available.
-
Ivermectin resistance mechanisms in ectoparasites: a scoping review.Parasitol Res. 2024 May 24;123(5):221. doi: 10.1007/s00436-024-08223-z. Parasitol Res. 2024. PMID: 38787430 Free PMC article.
-
Efficacy of ivermectin and its metabolites against Plasmodium falciparum liver stages in primary human hepatocytes.Antimicrob Agents Chemother. 2024 Aug 7;68(8):e0127223. doi: 10.1128/aac.01272-23. Epub 2024 Jun 21. Antimicrob Agents Chemother. 2024. PMID: 38904389 Free PMC article.
-
Lethal and sublethal impacts of membrane-fed ivermectin are concentration dependent in Anopheles coluzzii.Parasit Vectors. 2024 May 16;17(1):228. doi: 10.1186/s13071-024-06287-5. Parasit Vectors. 2024. PMID: 38755640 Free PMC article.
References
-
- WHO. World malaria report 2021. Geneva, World Health Organization, 2021. https://www.who.int/publications-detail-redirect/9789240040496. Accessed 8 Dec 2021.
-
- WHO. Tailoring malaria interventions in the COVID-19 response. Geneva, World Health Organization, 2020. http://www.who.int/malaria/publications/atoz/tailoring-malaria-intervent.... Accessed 14 May 2020.

