The HIV-1 capsid core is an opportunistic nuclear import receptor
- PMID: 37355754
- PMCID: PMC10290713
- DOI: 10.1038/s41467-023-39146-5
The HIV-1 capsid core is an opportunistic nuclear import receptor
Abstract
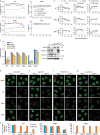
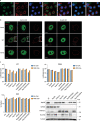
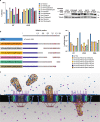
The movement of viruses and other large macromolecular cargo through nuclear pore complexes (NPCs) is poorly understood. The human immunodeficiency virus type 1 (HIV-1) provides an attractive model to interrogate this process. HIV-1 capsid (CA), the chief structural component of the viral core, is a critical determinant in nuclear transport of the virus. HIV-1 interactions with NPCs are dependent on CA, which makes direct contact with nucleoporins (Nups). Here we identify Nup35, Nup153, and POM121 to coordinately support HIV-1 nuclear entry. For Nup35 and POM121, this dependence was dependent cyclophilin A (CypA) interaction with CA. Mutation of CA or removal of soluble host factors changed the interaction with the NPC. Nup35 and POM121 make direct interactions with HIV-1 CA via regions containing phenylalanine glycine motifs (FG-motifs). Collectively, these findings provide additional evidence that the HIV-1 CA core functions as a macromolecular nuclear transport receptor (NTR) that exploits soluble host factors to modulate NPC requirements during nuclear invasion.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
G.X. is an employee of AstraZeneca and has stock ownership and/or stock options or interests in the company. The remaining authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

