Observed Improvement in Cognition During a Personalized Lifestyle Intervention in People with Cognitive Decline
- PMID: 37355891
- PMCID: PMC10473097
- DOI: 10.3233/JAD-230004
Observed Improvement in Cognition During a Personalized Lifestyle Intervention in People with Cognitive Decline
Abstract
Background: Alzheimer's disease (AD) is a chronic condition marked by progressive objective cognitive impairment (OCI). No monotherapy has substantially altered disease progression, suggesting the disease is multifactorial and may require a multimodal therapeutic approach.
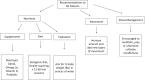
Objective: We sought to determine if cognitive function in a sample with OCI would change in response to a multimodal, individualized care plan based on potential contributors to cognitive decline (e.g., nutritional status, infection, etc.).
Methods: Participants (n = 34) were recruited from the San Diego, CA area. The multimodal intervention included lifestyle changes (i.e., movement, diet, and stress management), nutraceutical support, and medications. It was delivered pragmatically over four clinical visits, and outcome measures were gathered at four study visits, occurring at baseline, one, three, and six months (primary endpoint). Study participants received weekly phone calls for nutrition support throughout study participation. Outcome measures included the Cambridge Brain Sciences (CBS) battery, and the Montreal Cognitive Assessment (MoCA).
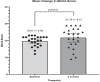
Results: At 6 months, mean MoCA scores improved from 19.6±3.1 to 21.7±6.2 (p = 0.013). Significant improvement was observed in mean scores of the CBS memory domain [25.2 (SD 23.3) to 35.8 (SD 26.9); p < 0.01] and CBS overall composite cognition score [24.5 (SD 16.1) to 29.7 (SD 20.5); p = 0.02]. All CBS domains improved.
Conclusion: Multiple measures of cognitive function improved after six months of intervention. Our results support the feasibility and impact of a multimodal, individualized treatment approach to OCI, warranting further research.
Keywords: Alzheimer’s disease; Cambridge Brain Sciences; Montreal Cognitive Assessment; clinical trial; dementia; mild cognitive impairment.
Conflict of interest statement
This manuscript is not under consideration by another journal, nor has it been published. Dr. Heather Sandison is the owner of a medical clinic and senior living facility. All the other authors have no conflict of interest to report.
Figures
References
-
- (2022) 2022 Alzheimer’s disease facts and figures. Alzheimers Dement 18, 700–789. - PubMed
-
- Wong W (2020) Economic burden of Alzheimer disease and managed care considerations. Am J Manag Care 26, S177–S183. - PubMed
-
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues JF, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert MO, Holtzman DM, Kivipelto M, Lista S, Molinuevo JL, O’Bryant SE, Rabinovici GD, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J, Jack CR Jr., Proceedings of the Meeting of the International Working Group (IWG) and the American Alzheimer’s Association on “The Preclinical State of AD”; July 23, 2015; Washington DC, USA (2016) Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement 12, 292–323. - PMC - PubMed

