Bone Marrow-Derived Mononuclear Cells in the Treatment of Neurological Diseases: Knowns and Unknowns
- PMID: 37356043
- PMCID: PMC11410020
- DOI: 10.1007/s10571-023-01377-x
Bone Marrow-Derived Mononuclear Cells in the Treatment of Neurological Diseases: Knowns and Unknowns
Abstract
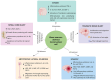
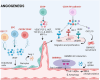
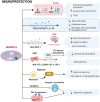
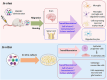
Bone marrow-derived mononuclear cells (BMMNCs) have been used for decades in preclinical and clinical studies to treat various neurological diseases. However, there is still a knowledge gap in the understanding of the underlying mechanisms of BMMNCs in the treatment of neurological diseases. In addition, prerequisite factors for the efficacy of BMMNC administration, such as the optimal route, dose, and number of administrations, remain unclear. In this review, we discuss known and unknown aspects of BMMNCs, including the cell harvesting, administration route and dose; mechanisms of action; and their applications in neurological diseases, including stroke, cerebral palsy, spinal cord injury, traumatic brain injury, amyotrophic lateral sclerosis, autism spectrum disorder, and epilepsy. Furthermore, recommendations on indications for BMMNC administration and the advantages and limitations of BMMNC applications for neurological diseases are discussed. BMMNCs in the treatment of neurological diseases. BMMNCs have been applied in several neurological diseases. Proposed mechanisms for the action of BMMNCs include homing, differentiation and paracrine effects (angiogenesis, neuroprotection, and anti-inflammation). Further studies should be performed to determine the optimal cell dose and administration route, the roles of BMMNC subtypes, and the indications for the use of BMMNCs in neurological conditions with and without genetic abnormalities.
Keywords: Administration route; Bone marrow-derived mononuclear cells; Cell therapy; Mechanism of action; Neurological diseases.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors have no relevant financial or nonfinancial interests to disclose.
Figures
References
-
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla AJN (2003) Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425(6961):968–973 - PubMed
-
- Amar AP, Zlokovic BV, Apuzzo MLJN (2003) Endovascular restorative neurosurgery: a novel concept for molecular and cellular therapy of the nervous system. Neurosurgery 52(2):402–413 - PubMed
-
- Anderson L, Burnstein RM, He X, Luce R, Furlong R, Foltynie T, Sykacek P, Menon DK (2007) Gene expression changes in long term expanded human neural progenitor cells passaged by chopping lead to loss of neurogenic potential in vivo. Exp Neurol 204(2):512–524 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

