Patterns of Prescription Medication Use during the First Trimester of Pregnancy in the United States, 1997-2018
- PMID: 37356083
- PMCID: PMC10949220
- DOI: 10.1002/cpt.2981
Patterns of Prescription Medication Use during the First Trimester of Pregnancy in the United States, 1997-2018
Abstract
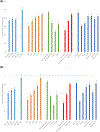
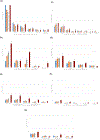
The objective of this analysis was to describe patterns of prescription medication use during pregnancy, including secular trends, with consideration of indication, and distributions of use within demographic subgroups. We conducted a descriptive secondary analysis using data from 9,755 women whose infants served as controls in two large United States case-control studies from 1997-2011 and 2014-2018. After excluding vitamin, herbal, mineral, vaccine, i.v. fluid, and topical products and over-the-counter medications, the proportion of women that reported taking at least one prescription medication in the first trimester increased over the study years, from 37% to 50% of women. The corresponding proportions increased with increasing maternal age and years of education, were highest for non-Hispanic White women (47%) and lowest for Hispanic women (24%). The most common indication for first trimester use of a medication was infection (12-15%). Increases were observed across the years for medications used for indications related to nausea/vomiting, depression/anxiety, infertility, thyroid disease, diabetes, and epilepsy. The largest relative increase in use among women was observed for medications to treat nausea/vomiting, which increased from 3.8% in the earliest years of the study (1997-2001) to 14.8% in 2014-2018, driven in large part by ondansetron use. Prescription medication use in the first trimester of pregnancy is common and increasing. Many medical conditions require treatments among pregnant women, often involving pharmacotherapy, which necessitates consideration of the risk and safety profiles for both mother and fetus.
© 2023 The Authors. Clinical Pharmacology & Therapeutics © 2023 American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
CONFLICT OF INTEREST
Dr. Mitchell is a member of the Pregnancy Advisory Board for Biogen’s Tecfidera Pregnancy Registry. Dr. Werler serves as a diagnostic adjudicator for Novartis pregnancy registries. All other authors declared no competing interests for this work.
Figures
References
-
- Lenz W A short history of thalidomide embryopathy. Teratology 38, 203–215 (1988). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

