Pathology explains various mechanisms of auto-immune inflammatory peripheral neuropathies
- PMID: 37356965
- PMCID: PMC10901618
- DOI: 10.1111/bpa.13184
Pathology explains various mechanisms of auto-immune inflammatory peripheral neuropathies
Abstract
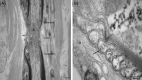
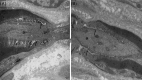
Autoimmune neuropathies are a heterogeneous group of rare and disabling diseases in which the immune system is thought to target antigens in the peripheral nervous system: they usually respond to immune therapies. Guillain-Barré syndrome is divided into several subtypes including "acute inflammatory demyelinating polyradiculoneuropathy," "acute motor axonal neuropathy," "acute motor sensory neuropathy," and other variants. Chronic forms such as chronic inflammatory demyelinating polyneuropathy (CIDP) and other subtypes and polyneuropathy associated with IgM monoclonal gammopathy; autoimmune nodopathies also belong to this group of auto-immune neuropathies. It has been shown that immunoglobulin G from the serum of about 30% of CIDP patients immunolabels nodes of Ranvier or paranodes of myelinated axons. Whatever the cause of myelin damage of the peripheral nervous system, the initial attack on myelin by a dysimmune process may begin either at the internodal area or in the paranodal and nodal regions. The term "nodoparanodopathy" was first applied to some "axonal Guillain-Barré syndrome" subtypes, then extended to cases classified as CIDP bearing IgG4 antibodies against paranodal axoglial proteins. In these cases, paranodal dissection develops in the absence of macrophage-induced demyelination. In contrast, the mechanisms of demyelination of other dysimmune neuropathies induced by macrophages are unexplained, as no antibodies have been identified in such cases. The main objective of this presentation is to show that the pathology illustrates, confirms, and may explain such mechanisms.
Keywords: internode; myelin; neurofascin; node; nodoparanodopathy; paranode.
© 2023 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

