Development and Validation of txSim: A Model of Advanced Lung Cancer Treatment in Australia
- PMID: 37357233
- PMCID: PMC10570197
- DOI: 10.1007/s40273-023-01291-6
Development and Validation of txSim: A Model of Advanced Lung Cancer Treatment in Australia
Abstract
Background and objective: Since 2016, new therapies have transformed the standard of care for lung cancer, creating a need for up-to-date evidence for health economic modelling. We developed a discrete event simulation of advanced lung cancer treatment to provide estimates of survival outcomes and healthcare costs in the Australian setting that can be updated as new therapies are introduced.
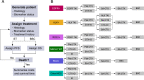
Methods: Treatment for advanced lung cancer was modelled under a clinician-specified treatment algorithm for Australia in 2022. Prevalence of lung cancer subpopulations was extracted from cBioPortal and the Sax Institute's 45 and Up Study, a large prospective cohort linked to cancer registrations. All costs were from the health system perspective for the year 2020. Pharmaceutical and molecular diagnostic costs were obtained from public reimbursement fees, while other healthcare costs were obtained from health system costs in the 45 and Up Study. Treatment efficacy was obtained from clinical trials and observational study data. Costs and survival were modelled over a 10-year horizon. Uncertainty intervals were generated with probabilistic sensitivity analyses. Overall survival predictions were validated against real-world studies.
Results: Under the 2022 treatment algorithm, estimated mean survival and costs for advanced lung cancer 10 years post-diagnosis were 16.4 months (95% uncertainty interval [UI]: 14.7-18.1) and AU$116,069 (95% UI: $107,378-$124,933). Survival and costs were higher assuming optimal treatment utilisation rates (20.5 months, 95% UI: 19.1-22.5; $154,299, 95% UI: $146,499-$161,591). The model performed well in validation, with good agreement between predicted and observed survival in real-world studies.
Conclusions: Survival improvements for advanced lung cancer have been accompanied by growing treatment costs. The estimates reported here can be used for budget planning and economic evaluations of interventions across the spectrum of cancer control.
© 2023. The Author(s).
Conflict of interest statement
Karen Canfell is a co-principal investigator of an investigator-initiated trial of cervical screening, “Compass”, run by the Australian Centre for Prevention of Cervical Cancer (ACPCC), which is a government-funded not-for-profit charity. Compass receives infrastructure support from the Australian government and the ACPCC has received equipment and a funding contribution from Roche Molecular Diagnostics, USA. Karen Canfell is also a co-principal investigator on a major implementation program Elimination of Cervical Cancer in the Western Pacific, which has received support from the Minderoo Foundation and the Frazer Family Foundation and equipment donations from Cepheid Inc. Zarnie Lwin declares participation on advisory boards/scientific advisory committees for, travel support from, and/or honoraria from Pfizer, AstraZeneca, Bristol Myers Squibb and Merck. Kwun M. Fong, David Goldsbury, Brett G.M. Hughes, Deme Karikios, Preston Ngo, Marianne Weber and Stephen Wade have no conflicts of interest that are directly relevant to the content of this article.
Figures


References
-
- Access Economics. Cost of cancer in NSW: a report by Access Economics Pty Limited for The Cancer Council NSW (2007). Cancer Council NSW. https://www.cancercouncil.com.au/wp-content/uploads/2010/11/costofcancer....
-
- Medical Services Advisory Committee. Application No. 1699 National Lung Cancer Screening Program; 2022. http://www.msac.gov.au/internet/msac/publishing.nsf/Content/1699-public [Accessed 8 Jun 2023].
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

