Mesenchymal progenitor cells from non-inflamed versus inflamed synovium post-ACL injury present with distinct phenotypes and cartilage regeneration capacity
- PMID: 37357305
- PMCID: PMC10291760
- DOI: 10.1186/s13287-023-03396-3
Mesenchymal progenitor cells from non-inflamed versus inflamed synovium post-ACL injury present with distinct phenotypes and cartilage regeneration capacity
Abstract
Background: Osteoarthritis (OA) is a chronic debilitating disease impacting a significant percentage of the global population. While there are numerous surgical and non-invasive interventions that can postpone joint replacement, there are no current treatments which can reverse the joint damage occurring during the pathogenesis of the disease. While many groups are investigating the use of stem cell therapies in the treatment of OA, we still don't have a clear understanding of the role of these cells in the body, including heterogeneity of tissue resident adult mesenchymal progenitor cells (MPCs).
Methods: In the current study, we examined MPCs from the synovium and individuals with or without a traumatic knee joint injury and explored the chondrogenic differentiation capacity of these MPCs in vitro and in vivo.
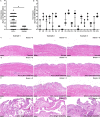
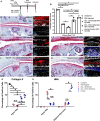
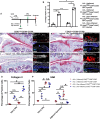
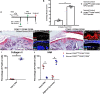
Results: We found that there is heterogeneity of MPCs with the adult synovium and distinct sub-populations of MPCs and the abundancy of these sub-populations change with joint injury. Furthermore, only some of these sub-populations have the ability to effect cartilage repair in vivo. Using an unbiased proteomics approach, we were able to identify cell surface markers that identify this pro-chondrogenic MPC population in normal and injured joints, specifically CD82LowCD59+ synovial MPCs have robust cartilage regenerative properties in vivo.
Conclusions: The results of this study clearly show that cells within the adult human joint can impact cartilage repair and that these sub-populations exist within joints that have undergone a traumatic joint injury. Therefore, these populations can be exploited for the treatment of cartilage injuries and OA in future clinical trials.
Keywords: ACL injury; Inflammation; Mesenchymal progenitor cells; Osteoarthritis.
© 2023. The Author(s).
Conflict of interest statement
Authors declare that they have no competing interests.
Figures



References
-
- Chahal J, Gómez-Aristizábal A, Shestopaloff K, Bhatt S, Chaboureau A, Fazio A, et al. Bone marrow mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Stem Cells Transl Med. 2019;8(8):746–757. doi: 10.1002/sctm.18-0183. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

