Deficiency for scavenger receptors Stabilin-1 and Stabilin-2 leads to age-dependent renal and hepatic depositions of fasciclin domain proteins TGFBI and Periostin in mice
- PMID: 37357460
- PMCID: PMC10497815
- DOI: 10.1111/acel.13914
Deficiency for scavenger receptors Stabilin-1 and Stabilin-2 leads to age-dependent renal and hepatic depositions of fasciclin domain proteins TGFBI and Periostin in mice
Abstract
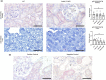
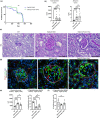
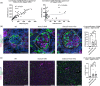
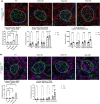
Stabilin-1 (Stab1) and Stabilin-2 (Stab2) are two major scavenger receptors of liver sinusoidal endothelial cells that mediate removal of diverse molecules from the plasma. Double-knockout mice (Stab-DKO) develop impaired kidney function and a decreased lifespan, while single Stabilin deficiency or therapeutic inhibition ameliorates atherosclerosis and Stab1-inhibition is subject of clinical trials in immuno-oncology. Although POSTN and TFGBI have recently been described as novel Stabilin ligands, the dynamics and functional implications of these ligands have not been comprehensively studied. Immunofluorescence, Western Blotting and Simple Western™ as well as in situ hybridization (RNAScope™) and qRT-PCR were used to analyze transcription levels and tissue distribution of POSTN and TGFBI in Stab-KO mice. Stab-POSTN-Triple deficient mice were generated to assess kidney and liver fibrosis and function in young and aged mice. TGFBI and POSTN protein accumulated in liver tissue in Stab-DKO mice and age-dependent in glomeruli of Stabilin-deficient mice despite unchanged transcriptional levels. Stab-POSTN-Triple KO mice showed glomerulofibrosis and a reduced lifespan comparable to Stab-DKO mice. However, alterations of the glomerular diameter and vascular density were partially normalized in Stab-POSTN-Triple KO. TGFBI and POSTN are Stabilin-ligands that are deposited in an age-dependent manner in the kidneys and liver due to insufficient scavenging in the liver. Functionally, POSTN might partially contribute to the observed renal phenotype in Stab-DKO mice. This study provides details on downstream effects how Stabilin dysfunction affects organ function on a molecular and functional level.
Keywords: POSTN; TGFBI; aging; fasciclin; lifespan; mouse; scavenger receptors.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures


References
-
- An, J. N. , Kim, H. , Kim, E. N. , Cho, A. , Cho, Y. , Choi, Y. W. , Kim, J. H. , Yang, S. H. , Choi, B. S. , Lim, C. S. , Kim, Y. S. , Kim, K. P. , & Lee, J. P. (2021). Effects of periostin deficiency on kidney aging and lipid metabolism. Aging (Albany NY), 13(19), 22649–22665. 10.18632/aging.203580 - DOI - PMC - PubMed
-
- An, J. N. , Yang, S. H. , Kim, Y. C. , Hwang, J. H. , Park, J. Y. , Kim, D. K. , Kim, J. H. , Kim, D. W. , Hur, D. G. , Oh, Y. K. , Lim, C. S. , Kim, Y. S. , & Lee, J. P. (2019). Periostin induces kidney fibrosis after acute kidney injury via the p38 MAPK pathway. American Journal of Physiology. Renal Physiology, 316(3), F426–f437. 10.1152/ajprenal.00203.2018 - DOI - PubMed
-
- Billings, P. C. , Whitbeck, J. C. , Adams, C. S. , Abrams, W. R. , Cohen, A. J. , Engelsberg, B. N. , Howard, P. S. , & Rosenbloom, J. (2002). The transforming growth factor‐β‐inducible matrix protein βig‐h3 interacts with fibronectin. Journal of Biological Chemistry, 277(31), 28003–28009. 10.1074/jbc.M106837200 - DOI - PubMed
-
- Guerrot, D. , Dussaule, J. C. , Mael‐Ainin, M. , Xu‐Dubois, Y. C. , Rondeau, E. , Chatziantoniou, C. , & Placier, S. (2012). Identification of periostin as a critical marker of progression/reversal of hypertensive nephropathy. PLoS One, 7(3), e31974. 10.1371/journal.pone.0031974 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

