Stapled β-Hairpin Antimicrobial Peptides with Improved Stability and Activity against Drug-Resistant Gram-Negative Bacteria
- PMID: 37357499
- PMCID: PMC10350921
- DOI: 10.1021/acs.jmedchem.3c00140
Stapled β-Hairpin Antimicrobial Peptides with Improved Stability and Activity against Drug-Resistant Gram-Negative Bacteria
Abstract
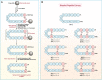
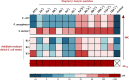
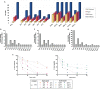
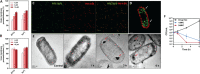
Different stapling techniques have been used recently to address the subpar performance of antimicrobial peptides (AMPs) in clinical trials with ample focus on α-helical AMPs. In comparison, a systematic evaluation of such strategies on β-hairpin AMPs is lacking. Herein, we report the design, synthesis, and evaluation of a library of all-hydrocarbon-stapled β-hairpin AMPs with variation in key parameters intended as potent therapeutics against drug-resistant pathogens. We observed an interesting interplay between the activity, stability, and structural strength. Single-stapled peptides with a 6-carbon staple at peptide termini such as 5(c6) displayed the most potent activity against colistin-resistant clinical isolates. Using imaging techniques, we observed translocation of 5(c6) across bacterial membranes without causing extensive damage. Overall, we have engineered novel all-hydrocarbon-stapled β-hairpin AMPs with structural and functional proficiency that can effectively combat resistant pathogens, with findings from this study a point of reference for future interests in developing novel β-hairpin AMPs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

