Efficacy and tolerability of an oral supplement containing amino acids, iron, selenium, and marine hydrolyzed collagen in subjects with hair loss (androgenetic alopecia, AGA or FAGA or telogen effluvium). A prospective, randomized, 3-month, controlled, assessor-blinded study
- PMID: 37357646
- PMCID: PMC10240182
- DOI: 10.1111/srt.13381
Efficacy and tolerability of an oral supplement containing amino acids, iron, selenium, and marine hydrolyzed collagen in subjects with hair loss (androgenetic alopecia, AGA or FAGA or telogen effluvium). A prospective, randomized, 3-month, controlled, assessor-blinded study
Abstract
Background: Oral supplementation with some amino acids (like methionine, taurine, and cysteine) could be useful in subjects with hair loss conditions such as androgenic alopecia (AGA or FAGA) or telogen effluvium (TE). Hydrolysed collagen (HC) oral supplementation has demonstrated to have beneficial effects on nail and skin health and could improve hair growth. A food supplement in tablet formulation containing hydrolysed fish-origin collagen (300 mg/dose), taurine, cysteine, methionine, iron, and selenium has been recently available. To date no controlled data are available regarding the clinical efficacy of this product as adjuvant to hair loss specific treatments in these clinical conditions.
Study aims: To evaluate and compare the efficacy and tolerability of an oral supplementation based on HC and amino acids in subjects with hair loss due to AGA/FAGA or chronic TE in combination with drug treatments in comparison with drug treatments alone.
Methods and subjects: In a prospective, 12-week, randomized, assessor-blinded controlled trial 83 subjects (mean age 41 ± 16 years; 26 men and 57 women) were enrolled in the study. Fifty-nine subjects suffered from AGA/FAGA (Hamilton I-VA, Ludwig I-1, II-2) and 24 from chronic TE. Subjects were randomized to oral supplementation (1 tablet day) in combination with the specify drug treatment decided by the investigator according to the type of hair loss (AGA/FAGA or TE) (Group A; N = 48) or to specific drugs treatment only (Group B; N = 35). The main outcome of the trial was the clinical efficacy evaluation using a 7-point global assessment score (GAS) (from +3: Much Improved to -3 Much worsened; with score 0 representing no modification). The GAS score was evaluated using standardized photographs by an investigator unaware of the treatment groups at week 6 and at week 12. A secondary outcome was the evaluation of acceptability of the treatment regimen using a 10-point evaluation score.
Results: Seventy-six participants (91.6%) completed the 12-week study period. The GAS score at week 6 was 0.5 ± 0.2 in group A and 0.0 ± 0.1 in Group B (p < 0.05; Mann-Whitney). At week 12 the GAS score in Group A was statistically significant higher in comparison with Group B (1.67 ± 0.16 and 0.66 ± 0.20, p < 0.001; Mann-Whitney test). A higher percentage of Group A subjects achieved a GAS score of ≥2 in comparison with group B (50% vs. 23%). The oral supplement was generally well tolerated.
Conclusion: An oral supplement containing hydrolysed fish-origin collagen, taurine, cysteine, methionine, iron, and selenium has demonstrated to improve the clinical efficacy of specific anti-hair loss treatments in subjects with AGA/FAGA or chronic TE.
Keywords: androgenic alopecia; hydrolysed fish-origin collagen; oral supplementation; telogen effluvium.
© 2023 The Authors. Skin Research and Technology published by John Wiley & Sons Ltd.
Conflict of interest statement
MM and FC are employees of Difa Cooper Catabria Labs that commercialized the food supplement. The authors report no other conflict of interest in this work.
Figures
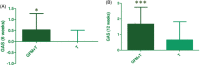



References
-
- Starace Michela, Milani Massimo, Piraccini Bianca Maria. Efficacy and tolerability of a topical gel containing mimicking growth factors oligopeptides, caffeine, taurine and an iron‐chelating complex in subjects with androgenetic alopecia treated with topical minoxidil or oral finasteride: a prospective, 6‐month, randomized, assessor‐blinded, 4‐Arm, Parallel Group Study. Clin Exp Dermatol Ther. 2022;7(4). doi:10.29011/2575-8268.100199 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

