Impact of Supplementary Immunization Activities using Novel Oral Polio Vaccine Type 2 during a Large outbreak of Circulating Vaccine-Derived Poliovirus in Nigeria
- PMID: 37357964
- PMCID: PMC10938209
- DOI: 10.1093/infdis/jiad222
Impact of Supplementary Immunization Activities using Novel Oral Polio Vaccine Type 2 during a Large outbreak of Circulating Vaccine-Derived Poliovirus in Nigeria
Abstract
Background: Novel oral poliovirus vaccine (OPV) type 2 (nOPV2) has been made available for outbreak response under an emergency use listing authorization based on supportive clinical trial data. Since 2021 more than 350 million doses of nOPV2 were used for control of a large outbreak of circulating vaccine-derived poliovirus type 2 (cVDPV2) in Nigeria.
Methods: Using a bayesian time-series susceptible-infectious-recovered model, we evaluate the field effectiveness of nOPV2 immunization campaigns in Nigeria compared with campaigns using monovalent OPV type 2 (mOPV2).
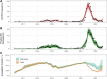
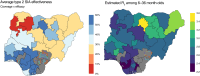
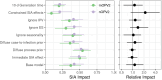
Results: We found that both nOPV2 and mOPV2 campaigns were highly effective in reducing transmission of cVDPV2, on average reducing the susceptible population by 42% (95% confidence interval, 28-54%) and 38% (20-51%) per campaign, respectively, which were indistinguishable from each other in this analysis (relative effect, 1.1 [.7-1.9]). Impact was found to vary across areas and between immunization campaigns.
Conclusions: These results are consistent with the comparable individual immunogenicity of nOPV2 and mOPV2 found in clinical trials but also suggest that outbreak response campaigns may have small impacts in some areas requiring more campaigns than are suggested in current outbreak response procedures.
Keywords: Nigeria; mass vaccination; oral poliovirus vaccine; poliovirus; susceptible infected recovered models.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. V., H. L., A. S. B., and S. O. are employed by the Bill & Melinda Gates Foundation. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Garon J, Seib K, Orenstein WA, et al. Polio endgame: the global switch from tOPV to bOPV. Expert Rev Vaccines 2016; 15:693–708. - PubMed
-
- Martin J, Burns CC, Jorba J, et al. Genetic characterization of novel oral polio vaccine type 2 viruses during initial use phase under emergency use listing—worldwide, March–October 2021. MMWR Morb Mortal Wkly Rep 2022; 71:786–90. - PubMed
-
- Bandyopadhyay AS, Zipursky S. A novel tool to eradicate an ancient scourge: the novel oral polio vaccine type 2 story. Lancet Infect Dis 2023; 23:e67–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

