Acyl coenzyme A binding protein (ACBP): An aging- and disease-relevant "autophagy checkpoint"
- PMID: 37357988
- PMCID: PMC10497816
- DOI: 10.1111/acel.13910
Acyl coenzyme A binding protein (ACBP): An aging- and disease-relevant "autophagy checkpoint"
Abstract
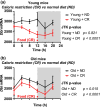
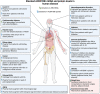
Acyl coenzyme A binding protein (ACBP), also known as diazepam-binding inhibitor (DBI), is a phylogenetically ancient protein present in some eubacteria and the entire eukaryotic radiation. In several eukaryotic phyla, ACBP/DBI transcends its intracellular function in fatty acid metabolism because it can be released into the extracellular space. This ACBP/DBI secretion usually occurs in response to nutrient scarcity through an autophagy-dependent pathway. ACBP/DBI and its peptide fragments then act on a range of distinct receptors that diverge among phyla, namely metabotropic G protein-coupled receptor in yeast (and likely in the mammalian central nervous system), a histidine receptor kinase in slime molds, and ionotropic gamma-aminobutyric acid (GABA)A receptors in mammals. Genetic or antibody-mediated inhibition of ACBP/DBI orthologs interferes with nutrient stress-induced adaptations such as sporulation or increased food intake in multiple species, as it enhances lifespan or healthspan in yeast, plant leaves, nematodes, and multiple mouse models. These lifespan and healthspan-extending effects of ACBP/DBI suppression are coupled to the induction of autophagy. Altogether, it appears that neutralization of extracellular ACBP/DBI results in "autophagy checkpoint inhibition" to unleash the anti-aging potential of autophagy. Of note, in humans, ACBP/DBI levels increase in various tissues, as well as in the plasma, in the context of aging, obesity, uncontrolled infection or cardiovascular, inflammatory, neurodegenerative, and malignant diseases.
Keywords: aging; autophagy; diazepam-binding inhibitor; endozepin; evolution; metabolism.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
GK has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Tollys, and Vascage. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific cofounder of everImmune, Osasuna Therapeutics, Samsara Therapeutics, and Therafast Bio. GK is in the scientific advisory boards of Hevolution, Institut Servier, and Longevity Vision Funds. GK is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis, and metabolic disorders. GK's wife, Laurence Zitvogel, has held research contracts with Glaxo Smyth Kline, Incyte, Lytix, Kaleido, Innovate Pharma, Daiichi Sankyo, Pilege, Merus, Transgene, 9 m, Tusk, and Roche, was on the on the Board of Directors of Transgene, is a cofounder of everImmune, and holds patents covering the treatment of cancer and the therapeutic manipulation of the microbiota. GK's brother, Romano Kroemer, was an employee of Sanofi and now consults for Boehringer‐Ingelheim. F.M. is a scientific cofounder of Samsara Therapeutics, and has equity interests in and is advisor of The Longevity Labs (TLL). LM, MA, OM, IM, and GK are listed as co‐inventors on ACBP/DBI‐relevant patents. The funders had no role in the design of the study, in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Abdellatif, M. , Sedej, S. , Carmona‐Gutierrez, D. , Madeo, F. , & Kroemer, G. (2018). Autophagy in cardiovascular aging. Circulation Research, 123, 803–824. - PubMed
-
- Abrahamsen, H. , & Stenmark, H. (2010). Protein secretion: unconventional exit by exophagy. Current Biology, 20, R415–R418. - PubMed
-
- Aman, Y. , Schmauck‐Medina, T. , Hansen, M. , Morimoto, R. I. , Simon, A. K. , Bjedov, I. , Palikaras, K. , Simonsen, A. , Johansen, T. , Tavernarakis, N. , Rubinsztein, D. C. , Partridge, L. , Kroemer, G. , Labbadia, J. , & Fang, E. F. (2021). Autophagy in healthy aging and disease. Nature Aging, 1, 634–650. - PMC - PubMed
-
- Anagnostopoulos, G. , Motino, O. , Li, S. , Carbonnier, V. , Chen, H. , Sica, V. , Durand, S. , Bourgin, M. , Aprahamian, F. , Nirmalathasan, N. , Donne, R. , Desdouets, C. , Sola, M. S. , Kotta, K. , Montégut, L. , Lambertucci, F. , Surdez, D. , Sandrine, G. , Delattre, O. , … Kroemer, G. (2022). An obesogenic feedforward loop involving PPARgamma, acyl‐CoA binding protein and GABAA receptor. Cell Death & Disease, 13, 356. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

