2023 European Thyroid Association Clinical Practice Guidelines for thyroid nodule management
- PMID: 37358008
- PMCID: PMC10448590
- DOI: 10.1530/ETJ-23-0067
2023 European Thyroid Association Clinical Practice Guidelines for thyroid nodule management
Abstract
With the widespread use of sensitive imaging techniques, which include neck visualization, a conspicuous number of thyroid nodules emerge and demand attention. Most lesions are benign, asymptomatic, and do not warrant treatment. In the case of cancer diagnosis, most are small, intrathyroidal and indolent neoplasms that can safely be managed conservatively. There is a pronounced need for more cost-effective, risk-adapted approaches to the management of this highly prevalent condition, taking the wishes of the patient into consideration. Thus, the present guidelines aim at providing a clinical practice guide for the initial workup and the subsequent management of adult individuals harboring thyroid nodules. Importantly, these guidelines are not intended to cover the management of thyroid malignancy. The manuscript and the specific recommendations were developed by reconciling the best available research evidence with the knowledge and clinical experience of the panelists and updating aspects of a number of previous European Thyroid Association guidelines.
Keywords: fine-needle aspiration; follow-up; management; minimally invasive treatment; molecular biology; surgery; thyroid nodule; treatment; ultrasound.
Conflict of interest statement
Agnieszka Czarniecka, Gilles Russ, Fernando Schmitt, Paula Soares, and Tamas Solymosi have no conflicts of interest to disclose. Cosimo Durante has reported advisory board honoraria from EISAI, Eli Lilly, and Roche. Laszlo Hegedüs has reported consultancy fees from Berlin-Chemie, Horizon, IBSA, Lundbeck, Merck-Serono, and Novo Nordisk Foundation. Enrico Papini has reported advisory board honoraria from IBSA and Terumo and consultancy fees from Novo Nordisk. Ralf Paschke has reported ThyroSPEC™ license fees, received grant from Bayer, and advisory board honoraria from Bayer, Eisai, and Ipsen.
Figures
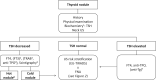
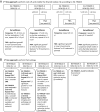
References
-
- Grani G, Zatelli MC, Alfò M, Montesano T, Torlontano M, Morelli S, Deandrea M, Antonelli A, Francese C, Ceresini G, et al. Real-world performance of the American Thyroid Association risk estimates in predicting 1-year differentiated thyroid cancer outcomes: a prospective multicenter study of 2000 patients. Thyroid 202131264–271. ( 10.1089/thy.2020.0272) - DOI - PubMed
-
- Lamartina L, Durante C, Lucisano G, Grani G, Bellantone R, Lombardi CP, Pontecorvi A, Arvat E, Felicetti F, Zatelli MC, et al. Are evidence-based guidelines reflected in clinical practice? An analysis of prospectively collected data of the Italian thyroid cancer observatory. Thyroid: Official Journal of the American Thyroid Association 2017271490–1497. ( 10.1089/thy.2017.0299) - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

