Reduction of class I histone deacetylases ameliorates ER-mitochondria cross-talk in Alzheimer's disease
- PMID: 37358017
- PMCID: PMC10410063
- DOI: 10.1111/acel.13895
Reduction of class I histone deacetylases ameliorates ER-mitochondria cross-talk in Alzheimer's disease
Abstract
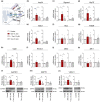
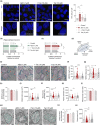
Several molecular mechanisms have been described in Alzheimer's disease (AD), including repressed gene transcription and mitochondrial and endoplasmic reticulum (ER) dysfunction. In this study, we evaluate the potential efficacy of transcriptional modifications exerted by inhibition or knockdown of class I histone deacetylases (HDACs) in ameliorating ER-mitochondria cross-talk in AD models. Data show increased HDAC3 protein levels and decreased acetyl-H3 in AD human cortex, and increased HDAC2-3 in MCI peripheral human cells, HT22 mouse hippocampal cells exposed to Aβ1-42 oligomers (AβO) and APP/PS1 mouse hippocampus. Tacedinaline (Tac, a selective class I HDAC inhibitor) counteracted the increase in ER-Ca2+ retention and mitochondrial Ca2+ accumulation, mitochondrial depolarization and impaired ER-mitochondria cross-talk, as observed in 3xTg-AD mouse hippocampal neurons and AβO-exposed HT22 cells. We further demonstrated diminished mRNA levels of proteins involved in mitochondrial-associated ER membranes (MAM) in cells exposed to AβO upon Tac treatment, along with reduction in ER-mitochondria contacts (MERCS) length. HDAC2 silencing reduced ER-mitochondria Ca2+ transfer and mitochondrial Ca2+ retention, while knockdown of HDAC3 decreased ER-Ca2+ accumulation in AβO-treated cells. APP/PS1 mice treated with Tac (30 mg/kg/day) also showed regulation of mRNA levels of MAM-related proteins, and reduced Aβ levels. These data demonstrate that Tac normalizes Ca2+ signaling between mitochondria and ER, involving the tethering between the two organelles in AD hippocampal neural cells. Tac-mediated AD amelioration occurs through the regulation of protein expression at MAM, as observed in AD cells and animal models. Data support transcriptional regulation of ER-mitochondria communication as a promising target for innovative therapeutics in AD.
Keywords: amyloid beta peptide; calcium; histone deacetylases; mitochondria; mitochondrial-associated ER membranes; tacedinaline.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no financial or non‐financial competing interests to report.
Figures




References
-
- Albert, M. S. , DeKosky, S. T. , Dickson, D. , Dubois, B. , Feldman, H. H. , Fox, N. C. , Gamst, A. , Holtzman, D. M. , Jagust, W. J. , Petersen, R. C. , Snyder, P. J. , Carrillo, M. C. , Thies, B. , & Phelps, C. H. (2011). The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia, 7(3), 270–279. 10.1016/j.jalz.2011.03.008 - DOI - PMC - PubMed
-
- Area‐Gomez, E. , Del Carmen Lara Castillo, M. , Tambini, M. D. , Guardia‐Laguarta, C. , de Groof, A. J. , Madra, M. , Ikenouchi, J. , Umeda, M. , Bird, T. D. , Sturley, S. L. , & Schon, E. A. (2012). Upregulated function of mitochondria‐associated ER membranes in Alzheimer disease. The EMBO Journal, 31(21), 4106–4123. 10.1038/emboj.2012.202 - DOI - PMC - PubMed
-
- Bartok, A. , Weaver, D. , Golenár, T. , Nichtova, Z. , Katona, M. , Bánsághi, S. , Alzayady, K. J. , Thomas, V. K. , Ando, H. , Mikoshiba, K. , Joseph, S. K. , Yule, D. I. , Csordás, G. , & Hajnóczky, G. (2019). IP3 receptor isoforms differently regulate ER‐mitochondrial contacts and local calcium transfer. Nature Communications, 10(1), 3726. 10.1038/s41467-019-11646-3 - DOI - PMC - PubMed
-
- Beaver, M. , Karisetty, B. C. , Zhang, H. , Bhatnagar, A. , Armour, E. , Parmar, V. , Brown, R. , Xiang, M. , & Elefant, F. (2022). Chromatin and transcriptomic profiling uncover dysregulation of the Tip60 HAT/HDAC2 epigenomic landscape in the neurodegenerative brain. Epigenetics, 17(7), 786–807. 10.1080/15592294.2021.1959742 - DOI - PMC - PubMed
-
- Beckers, T. , Burkhardt, C. , Wieland, H. , Gimmnich, P. , Ciossek, T. , Maier, T. , & Sanders, K. (2007). Distinct pharmacological properties of second generation HDAC inhibitors with the benzamide or hydroxamate head group. International Journal of Cancer, 121(5), 1138–1148. 10.1002/ijc.22751 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

