L-arginine metabolism as pivotal interface of mutual host-microbe interactions in the gut
- PMID: 37358082
- PMCID: PMC10294761
- DOI: 10.1080/19490976.2023.2222961
L-arginine metabolism as pivotal interface of mutual host-microbe interactions in the gut
Abstract
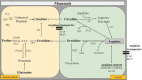
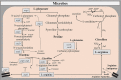
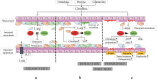
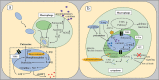
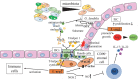
L-arginine (L-arg) is a versatile amino acid and a central intestinal metabolite in mammalian and microbial organisms. Thus, L-arg participates as precursor of multiple metabolic pathways in the regulation of cell division and growth. It also serves as a source of carbon, nitrogen, and energy or as a substrate for protein synthesis. Consequently, L-arg can simultaneously modify mammalian immune functions, intraluminal metabolism, intestinal microbiota, and microbial pathogenesis. While dietary intake, protein turnover or de novo synthesis usually supply L-arg in sufficient amounts, the expression of several key enzymes of L-arg metabolism can change rapidly and dramatically following inflammation, sepsis, or injury. Consequently, the availability of L-arg can be restricted due to increased catabolism, transforming L-arg into an essential amino acid. Here, we review the enzymatic pathways of L-arg metabolism in microbial and mammalian cells and their role in immune function, intraluminal metabolism, colonization resistance, and microbial pathogenesis in the gut.
Keywords: L-arginine (L-arg); colonization resistance; dietary L-arg supplementation; host–microbe interaction; intestinal microbiota; microbial pathogenesis; mucosal immune function; mutual metabolic pathways; virulence factor.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Dietary l-arginine supplementation ameliorates inflammatory response and alters gut microbiota composition in broiler chickens infected with Salmonella enterica serovar Typhimurium.Poult Sci. 2020 Apr;99(4):1862-1874. doi: 10.1016/j.psj.2019.10.049. Epub 2020 Mar 10. Poult Sci. 2020. PMID: 32241466 Free PMC article.
-
Glutamic acid supplementation reduces body fat weight in finishing pigs when provided solely or in combination with arginine and it is associated with colonic propionate and butyrate concentrations.Food Funct. 2019 Aug 1;10(8):4693-4704. doi: 10.1039/c9fo00520j. Epub 2019 Jul 12. Food Funct. 2019. PMID: 31298673
-
Dietary L-arginine supplementation enhances growth performance, intestinal antioxidative capacity, immunity and modulates gut microbiota in yellow-feathered chickens.Poult Sci. 2020 Dec;99(12):6935-6945. doi: 10.1016/j.psj.2020.09.042. Epub 2020 Sep 30. Poult Sci. 2020. PMID: 33248609 Free PMC article.
-
Succinate metabolism and its regulation of host-microbe interactions.Gut Microbes. 2023 Jan-Dec;15(1):2190300. doi: 10.1080/19490976.2023.2190300. Gut Microbes. 2023. PMID: 36946592 Free PMC article. Review.
-
Bile Acids and Microbiota: Multifaceted and Versatile Regulators of the Liver-Gut Axis.Int J Mol Sci. 2021 Jan 30;22(3):1397. doi: 10.3390/ijms22031397. Int J Mol Sci. 2021. PMID: 33573273 Free PMC article. Review.
Cited by
-
Host-microbiota interaction in intestinal stem cell homeostasis.Gut Microbes. 2024 Jan-Dec;16(1):2353399. doi: 10.1080/19490976.2024.2353399. Epub 2024 May 17. Gut Microbes. 2024. PMID: 38757687 Free PMC article. Review.
-
Optimization of citrulline production from a Bacillus subtilis BH-01 isolated from raw buffalo milk.BMC Microbiol. 2025 Feb 10;25(1):71. doi: 10.1186/s12866-025-03768-0. BMC Microbiol. 2025. PMID: 39930373 Free PMC article.
-
Lacticaseibacillus rhamnosus GG-driven remodeling of arginine metabolism mitigates gut barrier dysfunction.Am J Physiol Gastrointest Liver Physiol. 2025 Jul 1;329(1):G162-G185. doi: 10.1152/ajpgi.00366.2024. Epub 2025 May 26. Am J Physiol Gastrointest Liver Physiol. 2025. PMID: 40418622 Free PMC article.
-
Arginine at the host-pathogen interface.Infect Immun. 2025 Aug 12;93(8):e0061224. doi: 10.1128/iai.00612-24. Epub 2025 Jul 3. Infect Immun. 2025. PMID: 40607975 Free PMC article. Review.
-
Productive and metabolomic consequences of arginine supplementation in sows during different gestation periods in two different seasons.J Anim Sci Biotechnol. 2024 Sep 19;15(1):121. doi: 10.1186/s40104-024-01079-4. J Anim Sci Biotechnol. 2024. PMID: 39294768 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
