The role of pneumococcal extracellular vesicles on the pathophysiology of the kidney disease hemolytic uremic syndrome
- PMID: 37358300
- PMCID: PMC10449520
- DOI: 10.1128/msphere.00142-23
The role of pneumococcal extracellular vesicles on the pathophysiology of the kidney disease hemolytic uremic syndrome
Abstract
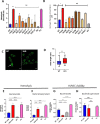
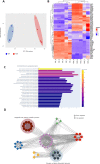
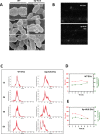
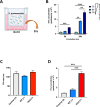
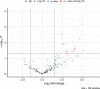
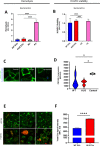
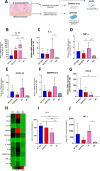
Streptococcus pneumoniae-induced hemolytic uremic syndrome (Sp-HUS) is a kidney disease characterized by microangiopathic hemolytic anemia, thrombocytopenia, and acute kidney injury. This disease is frequently underdiagnosed and its pathophysiology is poorly understood. In this work, we compared clinical strains, isolated from infant Sp-HUS patients, with a reference pathogenic strain D39, for host cytotoxicity and further explored the role of Sp-derived extracellular vesicles (EVs) in the pathogenesis of an HUS infection. In comparison with the wild-type strain, pneumococcal HUS strains caused significant lysis of human erythrocytes and increased the release of hydrogen peroxide. Isolated Sp-HUS EVs were characterized by performing dynamic light-scattering microscopy and proteomic analysis. Sp-HUS strain released EVs at a constant concentration during growth, yet the size of the EVs varied and several subpopulations emerged at later time points. The cargo of the Sp-HUS EVs included several virulence factors at high abundance, i.e., the ribosomal subunit assembly factor BipA, the pneumococcal surface protein A, the lytic enzyme LytC, several sugar utilization, and fatty acid synthesis proteins. Sp-HUS EVs strongly downregulated the expression of the endothelial surface marker platelet endothelial cell adhesion molecule-1 and were internalized by human endothelial cells. Sp-HUS EVs elicited the release of pro-inflammatory cytokines (interleukin [IL]-1β, IL-6) and chemokines (CCL2, CCL3, CXCL1) by human monocytes. These findings shed new light on the overall function of Sp-EVs, in the scope of infection-mediated HUS, and suggest new avenues of research for exploring the usefulness of Sp-EVs as therapeutic and diagnostic targets. IMPORTANCE Streptococcus pneumoniae-associated hemolytic uremic syndrome (Sp-HUS) is a serious and underdiagnosed deadly complication of invasive pneumococcal disease. Despite the introduction of the pneumococcal vaccine, cases of Sp-HUS continue to emerge, especially in children under the age of 2. While a lot has been studied regarding pneumococcal proteins and their role on Sp-HUS pathophysiology, little is known about the role of extracellular vesicles (EVs). In our work, we isolate and initially characterize EVs from a reference pathogenic strain (D39) and a strain isolated from a 2-year-old patient suffering from Sp-HUS. We demonstrate that despite lacking cytotoxicity toward human cells, Sp-HUS EVs are highly internalized by endothelial cells and can trigger cytokine and chemokine production in monocytes. In addition, this work specifically highlights the distinct morphological characteristics of Sp-HUS EVs and their unique cargo. Overall, this work sheds new light into potentially relevant players contained in EVs that might elucidate about pneumococcal EVs biogenesis or pose as interesting candidates for vaccine design.
Keywords: cytokines; extracellular vesicles; immunomodulation; microbe-host.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Meinel C, Spartà G, Dahse H-M, Hörhold F, König R, Westermann M, Coldewey SM, Cseresnyés Z, Figge MT, Hammerschmidt S, Skerka C, Zipfel PF. 2018. Streptococcus pneumoniae from patients with hemolytic uremic syndrome binds human plasminogen via the surface protein PspC and uses plasmin to damage human endothelial cells. J Infect Dis 217:358–370. doi: 10.1093/infdis/jix305 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
