Bacterial Swarm-Mediated Phage Transportation Disrupts a Biofilm Inherently Protected from Phage Penetration
- PMID: 37358420
- PMCID: PMC10434198
- DOI: 10.1128/spectrum.00937-23
Bacterial Swarm-Mediated Phage Transportation Disrupts a Biofilm Inherently Protected from Phage Penetration
Abstract
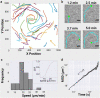
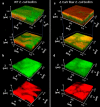
Physical forces that arise due to bacterial motility and growth play a significant role in shaping the biogeography of the human oral microbiota. Bacteria of the genus Capnocytophaga are abundant in the human oral microbiota and yet very little is known about their physiology. The human oral isolate Capnocytophaga gingivalis exhibits robust gilding motility that is driven by the rotary type 9 secretion system (T9SS), and cells of C. gingivalis transport nonmotile oral microbes as cargo. Phages, i.e., viruses that infect bacteria, are found in abundance within the microbiota. By tracking fluorescently labeled lambda phages that do not infect C. gingivalis, we report active phage transportation by C. gingivalis swarms. Lambda phage-carrying C. gingivalis swarms were propagated near an Escherichia coli colony. The rate of disruption of the E. coli colony increased 10 times compared with a control where phages simply diffused to the E. coli colony. This finding suggests a mechanism where fluid flows produced by motile bacteria increase the rate of transport of phages to their host bacterium. Additionally, C. gingivalis swarms formed tunnel-like structures within a curli fiber-containing E. coli biofilm that increased the efficiency of phage penetration. Our data suggest that invasion by a C. gingivalis swarm changes the spatial structure of the prey biofilm and further increases the penetration of phages. IMPORTANCE Dysbiosis of the human oral microbiota is associated with several diseases, but the factors that shape the biogeography of the oral microbiota are mostly opaque. Biofilms that form in the human supragingival and subgingival regions have a diverse microbial community where some microbes form well-defined polymicrobial structures. C. gingivalis, a bacterium abundant in human gingival regions, has robust gliding motility that is powered by the type 9 secretion system. We demonstrate that swarms of C. gingivalis can transport phages through a complex biofilm which increases the death rate of the prey biofilm. These findings suggest that C. gingivalis could be used as a vehicle for the transportation of antimicrobials and that active phage transportation could shape the spatial structure of a microbial community.
Keywords: active transport; bacterial motility; biofilm; collective motion; gliding motility; oral microbiome; phage; swarming; type 9 secretion system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Berg HC. 1993. Random Walks in Biology. Princeton University Press, New Jersey, USA. https://press.princeton.edu/books/paperback/9780691000640/random-walks-i.... Retrieved 15 June 2021.
-
- Cauda V, Onida B, Platschek B, Mühlstein L, Bein T. 2008. Large antibiotic molecule diffusion in confined mesoporous silica with controlled morphology. J Mater Chem 18:5888–5899. doi:10.1039/b805395b. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

