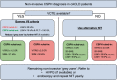
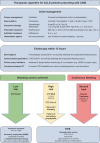
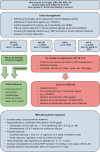
Austrian consensus on the diagnosis and management of portal hypertension in advanced chronic liver disease (Billroth IV)
- PMID: 37358642
- PMCID: PMC10319704
- DOI: 10.1007/s00508-023-02229-w
Austrian consensus on the diagnosis and management of portal hypertension in advanced chronic liver disease (Billroth IV)
Abstract
The Billroth IV consensus was developed during a consensus meeting of the Austrian Society of Gastroenterology and Hepatology (ÖGGH) and the Austrian Society of Interventional Radiology (ÖGIR) held on the 26th of November 2022 in Vienna.Based on international recommendations and considering recent landmark studies, the Billroth IV consensus provides guidance regarding the diagnosis and management of portal hypertension in advanced chronic liver disease.
Keywords: Acute-on-chronic liver failure; Ascites; Cirrhosis; Elastography; HVPG; Hepatorenal syndrome; Portal vein thrombosis; Spontaneous bacterial peritonitis; Transjugular intrahepatic portosystemic shunt; Variceal bleeding; Varices.
© 2023. The Author(s).
Conflict of interest statement
M. Mandorfer served as a speaker and/or consultant and/or advisory board member for AbbVie, Albireo, Collective Acumen, Gilead, Takeda, and W. L. Gore & Associates and received travel support from AbbVie and Gilead. E. Aigner served as a speaker and/or consultant and/or advisory board member for Gilead, Sanofi-Genzyme, Takeda, Advanz Pharma, Roche, Novartis. B. Scheiner received travel support fromm AbbVie, Ipsen and Gilead. M. Gschwantler received grants from AbbVie, Gilead, and MSD; speaking honoraria/advisory board fees from AbbVie, Gilead, MSD, Janssen, BMS, Roche, Intercept, Alnylam, Norgine, AstraZeneca, Falk, Gebro Pharma and Shionogi. M. Jachs served as a speaker and/or consultant for Gilead and received travel support from Gilead. S. Hametner-Schreil served as speaker and/or advisory board member for AbbVie, Gilead, Roche and MSD. M. Peck-Radosavljevic has served as an investigator for AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Exelixis, Eisai, Gilead, Lilly, Ipsen, Novartis, and Roche. He has served as speaker or adviser for AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Eisai, Gilead, Ipsen, Lilly, MSD, and Roche. He received grant support from Bayer and Gilead, and he served in data and safety monitoring boards for BMS, Boehringer Ingelheim, Lilly-Imclonse and ONEXO. P. Schwabl served as constultant for PharmaIN. R. Stauber served as a speaker for Boston Scientific and Medtronic. S. Reiter received speakers’ honoraria from Intercept Pharma Austria as well as travel support from AbbVie and Gilead. M. Trauner received grant support from Albireo, Alnylam, Cymabay, Falk, Gilead, Intercept, MSD, Takeda, and UltraGenyx, honoraria for consulting from Albireo, Boehringer Ingelheim, BiomX, Falk, Genfit, Gilead, Hightide, Intercept, Janssen, MSD, Novartis, Phenex, Pliant, Regulus, and Shire, speaker fees from Bristol-Myers Squibb, Falk, Gilead, Intercept, and MSD, as well as travel support from AbbVie, Falk, Gilead, Intercept and Jannsen. V. Stadlbauer received speaker’s honoraria and travel support from Merz Therapeutics, Albireo, Sanofi. T. Reiberger served as a speaker and/or consultant and/or advisory board member speaking honoraria from AbbVie, Bayer, Boehringer-Ingelheim, Gilead, Intercept, MSD, Roche, Siemens, and W. L. Gore & Associates and received travel support from AbbVie, Boehringer-Ingelheim, Gilead, and Roche as well as grants/research support from AbbVie, Boehringer-Ingelheim, Gilead, Intercept, MSD, Myr Pharmaceuticals, Philips Healthcare, Pliant, Siemens, and W. L. Gore & Associates. M. Cejna, A. Ferlitsch, C. Datz, T. Gräter, I. Graziadei, H. Hofer, A. Loizides, A. Maieron, F. Rainer, , G. Semmler, L. Reider, M. Schoder, R. Schöfl, E. Tatscher, A. Ziachehabi, H. Zoller and P. Fickert declare that they have no competing interests.
Figures
References
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. - DOI - PMC - PubMed
-
- Semmler G, Hartl L, Jachs M, Simbrunner B, Hofer BS, Balcar L, et al. EASL liver congress (TM) abstract # 2477: is elastography needed for diagnosing cACLD and stratifying CSPH risk? 2023.
MeSH terms
LinkOut - more resources
Full Text Sources

