Infection promotes Ser-214 phosphorylation important for generation of cytotoxic tau variants
- PMID: 37358817
- PMCID: PMC12306725
- DOI: 10.1096/fj.202300620RR
Infection promotes Ser-214 phosphorylation important for generation of cytotoxic tau variants
Abstract
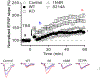
Patients who recover from hospital-acquired pneumonia exhibit a high incidence of end-organ dysfunction following hospital discharge, including cognitive deficits. We have previously demonstrated that pneumonia induces the production and release of cytotoxic oligomeric tau from pulmonary endothelial cells, and these tau oligomers can enter the circulation and may be a cause of long-term morbidities. Endothelial-derived oligomeric tau is hyperphosphorylated during infection. The purpose of these studies was to determine whether Ser-214 phosphorylation of tau is a necessary stimulus for generation of cytotoxic tau variants. The results of these studies demonstrate that Ser-214 phosphorylation is critical for the cytotoxic properties of infection-induced oligomeric tau. In the lung, Ser-214 phosphorylated tau contributes to disruption of the alveolar-capillary barrier, resulting in increased permeability. However, in the brain, both the Ser-214 phosphorylated tau and the mutant Ser-214-Ala tau, which cannot be phosphorylated, disrupted hippocampal long-term potentiation suggesting that inhibition of long-term potentiation was relatively insensitive to the phosphorylation status of Ser-214. Nonetheless, phosphorylation of tau is essential to its cytotoxicity since global dephosphorylation of the infection-induced cytotoxic tau variants rescued long-term potentiation. Collectively, these data demonstrate that multiple forms of oligomeric tau are generated during infectious pneumonia, with different forms of oligomeric tau being responsible for dysfunction of distinct end-organs during pneumonia.
Keywords: Pseudomonas aeruginosa; cytotoxicity; endothelium; long-term potentiation; oligomeric tau; pneumonia.
© 2023 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
DISCLOSURES
The authors declare no conflicts of interest.
Figures




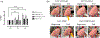
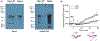
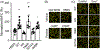
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

