A Digital Mental Health App Incorporating Wearable Biosensing for Teachers of Children on the Autism Spectrum to Support Emotion Regulation: Protocol for a Pilot Randomized Controlled Trial
- PMID: 37358908
- PMCID: PMC10337316
- DOI: 10.2196/45852
A Digital Mental Health App Incorporating Wearable Biosensing for Teachers of Children on the Autism Spectrum to Support Emotion Regulation: Protocol for a Pilot Randomized Controlled Trial
Abstract
Background: As much as 80% of children on the autism spectrum exhibit challenging behaviors (ie, behaviors dangerous to the self or others, behaviors that interfere with learning and development, and behaviors that interfere with socialization) that can have a devastating impact on personal and family well-being, contribute to teacher burnout, and even require hospitalization. Evidence-based practices to reduce these behaviors emphasize identifying triggers (events or antecedents that lead to challenging behaviors); however, parents and teachers often report that challenging behaviors surface with little warning. Exciting recent advances in biometric sensing and mobile computing technology allow the measurement of momentary emotion dysregulation using physiological indexes.
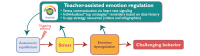
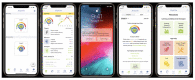
Objective: We present the framework and protocol for a pilot trial that will test a mobile digital mental health app, the KeepCalm app. School-based approaches to managing challenging behaviors in children on the autism spectrum are limited by 3 key factors: children on the autism spectrum often have difficulties in communicating their emotions; it is challenging to implement evidence-based, personalized strategies for individual children in group settings; and it is difficult for teachers to track which strategies are successful for each child. KeepCalm aims to address those barriers by communicating children's stress to their teachers using physiological signaling (emotion dysregulation detection), supporting the implementation of emotion regulation strategies via smartphone pop-up notifications of top strategies for each child according to their behavior (emotion regulation strategy implementation), and easing the task of tracking outcomes by providing the child's educational team with a tool to track the most effective emotion regulation strategies for that child based on physiological stress reduction data (emotion regulation strategy evaluation).
Methods: We will test KeepCalm with 20 educational teams of students on the autism spectrum with challenging behaviors (no exclusion based on IQ or speaking ability) in a pilot randomized waitlist-controlled field trial over a 3-month period. We will examine the usability, acceptability, feasibility, and appropriateness of KeepCalm as primary outcomes. Secondary preliminary efficacy outcomes include clinical decision support success, false positives or false negatives of stress alerts, and the reduction of challenging behaviors and emotion dysregulation. We will also examine technical outcomes, including the number of artifacts and the proportion of time children are engaged in high physical movement based on accelerometry data; test the feasibility of our recruitment strategies; and test the response rate and sensitivity to change of our measures, in preparation for a future fully powered large-scale randomized controlled trial.
Results: The pilot trial will begin by September 2023.
Conclusions: Results will provide key data about important aspects of implementing KeepCalm in preschools and elementary schools and will provide preliminary data about its efficacy to reduce challenging behaviors and support emotion regulation in children on the autism spectrum.
Trial registration: ClinicalTrials.gov NCT05277194; https://www.clinicaltrials.gov/ct2/show/NCT05277194.
International registered report identifier (irrid): PRR1-10.2196/45852.
Keywords: JITAI; autism; challenging behavior; digital mental health; emotion dysregulation; evidence-based strategies; heart rate tracking; just-in-time adaptive intervention augmentation; mobile phone; student progress monitoring.
©Emma H Palermo, Amanda V Young, Sky Deswert, Alyssa Brown, Miranda Goldberg, Evan Sultanik, Jessica Tan, Carla A Mazefsky, Lauren Brookman-Frazee, James C McPartland, Matthew S Goodwin, Jeffrey Pennington, Steven C Marcus, Rinad S Beidas, David S Mandell, Heather J Nuske. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 26.06.2023.
Conflict of interest statement
Conflicts of Interest: HJN consults with 2 digital health companies, Songbird Therapy and Pletly. CAM receives royalties from Oxford University Press and is on the scientific advisory board for the Brain and Behavior Foundation. JCM consults with Customer Value Partners, Bridgebio, Determined Health, and BlackThorn Therapeutics; has received research funding from Janssen Research and Development; serves on the Scientific Advisory Boards of Pastorus and Modern Clinics; and receives royalties from Guilford Press, Lambert, and Springer. RSB is the principal at Implementation Science & Practice, LLC. She receives royalties from Oxford University Press and consulting fees from United Behavioral Health and OptumLabs and serves on the advisory boards for Optum Behavioral Health, AIM Youth Mental Health Foundation, and the Klingenstein Third Generation Foundation. All are outside the scope of the submitted work. All other authors have no conflicts of interest to declare.
Figures
References
-
- Machalicek W, O’Reilly MF, Beretvas N, Sigafoos J, Lancioni GE. A review of interventions to reduce challenging behavior in school settings for students with autism spectrum disorders. Res Autism Spectrum Disord. 2007 Jul;1(3):229–46. doi: 10.1016/j.rasd.2006.10.005. - DOI
-
- Rojahn J, Matson J, Lott D, Esbensen A, Smalls Y. The Behavior Problems Inventory: an instrument for the assessment of self-injury, stereotyped behavior, and aggression/destruction in individuals with developmental disabilities. J Autism Dev Disord. 2001 Dec;31(6):577–88. doi: 10.1023/a:1013299028321. - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous