c-Abl tyrosine kinase down-regulation as target for memory improvement in Alzheimer's disease
- PMID: 37358955
- PMCID: PMC10289333
- DOI: 10.3389/fnagi.2023.1180987
c-Abl tyrosine kinase down-regulation as target for memory improvement in Alzheimer's disease
Abstract
Background: Growing evidence suggests that the non-receptor tyrosine kinase, c-Abl, plays a significant role in the pathogenesis of Alzheimer's disease (AD). Here, we analyzed the effect of c-Abl on the cognitive performance decline of APPSwe/PSEN1ΔE9 (APP/PS1) mouse model for AD.
Methods: We used the conditional genetic ablation of c-Abl in the brain (c-Abl-KO) and pharmacological treatment with neurotinib, a novel allosteric c-Abl inhibitor with high brain penetrance, imbued in rodent's chow.
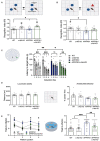
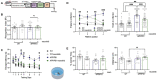
Results: We found that APP/PS1/c-Abl-KO mice and APP/PS1 neurotinib-fed mice had improved performance in hippocampus-dependent tasks. In the object location and Barnes-maze tests, they recognized the displaced object and learned the location of the escape hole faster than APP/PS1 mice. Also, APP/PS1 neurotinib-fed mice required fewer trials to reach the learning criterion in the memory flexibility test. Accordingly, c-Abl absence and inhibition caused fewer amyloid plaques, reduced astrogliosis, and preserved neurons in the hippocampus.
Discussion: Our results further validate c-Abl as a target for AD, and the neurotinib, a novel c-Abl inhibitor, as a suitable preclinical candidate for AD therapies.
Keywords: Alzheimer’s disease; Hippocampi; c-Abl inhibitors; memory; tyrosine kinases.
Copyright © 2023 León, Gutiérrez, Pinto, Morales, de la Fuente, Riquelme, Cortés, González-Martin, Chamorro, Espinosa, Fuentealba, Cancino, Zanlungo, Dulcey, Marugan and Álvarez Rojas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Neurotinib is under the patent WO2019/173761 A1.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

