Combining bacteriophage and vancomycin is efficacious against MRSA biofilm-like aggregates formed in synovial fluid
- PMID: 37359001
- PMCID: PMC10289194
- DOI: 10.3389/fmed.2023.1134912
Combining bacteriophage and vancomycin is efficacious against MRSA biofilm-like aggregates formed in synovial fluid
Abstract
Background: Biofilm formation is a major clinical challenge contributing to treatment failure of periprosthetic joint infection (PJI). Lytic bacteriophages (phages) can target biofilm associated bacteria at localized sites of infection. The aim of this study is to investigate whether combination therapy of phage and vancomycin is capable of clearing Staphylococcus aureus biofilm-like aggregates formed in human synovial fluid.
Methods: In this study, S. aureus BP043, a PJI clinical isolate was utilized. This strain is a methicillin-resistant S. aureus (MRSA) biofilm-former. Phage Remus, known to infect S. aureus, was selected for the treatment protocol. BP043 was grown as aggregates in human synovial fluid. The characterization of S. aureus aggregates was assessed for structure and size using scanning electron microscopy (SEM) and flow cytometry, respectively. Moreover, the formed aggregates were subsequently treated in vitro with: (a) phage Remus [∼108 plaque-forming units (PFU)/ml], (b) vancomycin (500 μg/ml), or (c) phage Remus (∼108 PFU/ml) followed by vancomycin (500 μg/ml), for 48 h. Bacterial survival was quantified by enumeration [colony-forming units (CFU)/ml]. The efficacy of phage and vancomycin against BP043 aggregates was assessed in vivo as individual treatments and in combination. The in vivo model utilized Galleria mellonella larvae which were infected with BP043 aggregates pre-formed in synovial fluid.
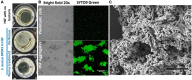
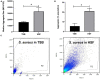
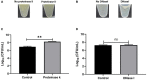
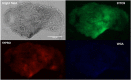
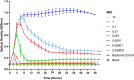
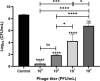
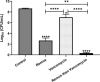
Results: Scanning electron microscopy (SEM) images and flow cytometry data demonstrated the ability of human synovial fluid to promote formation of S. aureus aggregates. Treatment with Remus resulted in significant reduction in viable S. aureus residing within the synovial fluid aggregates compared to the aggregates that did not receive Remus (p < 0.0001). Remus was more efficient in eliminating viable bacteria within the aggregates compared to vancomycin (p < 0.0001). Combination treatment of Remus followed by vancomycin was more efficacious in reducing bacterial load compared to using either Remus or vancomycin alone (p = 0.0023, p < 0.0001, respectively). When tested in vivo, this combination treatment also resulted in the highest survival rate (37%) 96 h post-treatment, compared to untreated larvae (3%; p < 0.0001).
Conclusion: We demonstrate that combining phage Remus and vancomycin led to synergistic interaction against MRSA biofilm-like aggregates in vitro and in vivo.
Keywords: Galleria mellonella; Staphylococcus aureus; aggregates; biofilm; peri-prosthetic joint infection; phage; synovial fluid; vancomycin.
Copyright © 2023 Taha, Arnaud, Lightly, Peters, Wang, Chen, Cook, Theriault and Abdelbary.
Conflict of interest statement
TA, TL, BC, and ST were employed by Cytophage Technologies Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Kunutsor S, Whitehouse M, Blom A, Board T, Kay P, Wroblewski B, et al. One-and two-stage surgical revision of peri-prosthetic joint infection of the hip: a pooled individual participant data analysis of 44 cohort studies. Eur J Epidemiol. (2018) 33:933–46. 10.1007/s10654-018-0377-9 - DOI - PMC - PubMed
-
- Hartzler M, Li K, Geary M, Odum S, Springer B. Complications in the treatment of prosthetic joint infection. Bone Joint J. (2020) 102:145–50. - PubMed
-
- Lenguerrand E, Whitehouse M, Beswick A, Toms A, Porter M, Blom A, et al. Description of the rates, trends and surgical burden associated with revision for prosthetic joint infection following primary and revision knee replacements in England and Wales: an analysis of the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. BMJ Open. (2017) 7:e014056. 10.1136/bmjopen-2016-014056 - DOI - PMC - PubMed
-
- Singh R, Ray P, Das A, Sharma M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother. (2010) 65:1955–8. - PubMed
LinkOut - more resources
Full Text Sources
Medical

