Safety of SARS-CoV-2 Vaccination in Patients with Vascular Malformations: Patient-Reported Adverse Vaccine Reactions
- PMID: 37359097
- PMCID: PMC10288124
- DOI: 10.3400/avd.oa.22-00126
Safety of SARS-CoV-2 Vaccination in Patients with Vascular Malformations: Patient-Reported Adverse Vaccine Reactions
Abstract
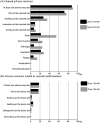
Objectives: Concerns among susceptible individuals, especially those with vascular malformations, have been raised by reports of thromboembolism following the administration of the SARS-CoV-2 vaccination against coronavirus disease 2019 (COVID-19). This study's goal was to assess any negative side effects that patients with vascular malformations who received the SARS-CoV-2 vaccine reported after receiving it. Materials and Methods: Through the three patient groups for vascular malformations in Japan in November 2021, a questionnaire was distributed to patients with vascular malformations who were 12 years of age or older. Multiple regression analysis was used to find relevant variables. Results: A total of 128 patients responded, representing a response rate of 58.8%. Ninety-six participants (75.0%) had received at least one dose of SARS-CoV-2 vaccine. In total, 84 (87.5%) and 84 (89.4%) subjects experienced at least 1 general adverse response following dose 1 and dose 2, respectively. Adverse reactions related to vascular malformations were reported by 15 participants (16.0%) after the 1st dose and 17 (17.7%) after the 2nd dose. Notably, no case of thromboembolism following vaccination was reported. Conclusion: The rate of vaccine-related adverse reactions in patients with vascular malformations is not different from that reported in the general population. There is no report of life-threatening responses in the research population.
Keywords: COVID-19; adverse reactions; vaccination; vascular anomalies; vascular malformations.
© 2023 The Editorial Committee of Annals of Vascular Diseases.
Conflict of interest statement
Disclosure StatementDeclaration of Conflicting Interests: The authors declared no conflicts of interest. No funding was received for conducting this research.
Figures
References
-
- Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 1982; 69: 412-20. doi: 10.1097/00006534-198203000-00002. - PubMed
-
- Enjolras O. Classification and management of the various superficial vascular anomalies: hemangiomas and vascular malformations. J Dermatol 1997; 24: 701-10. doi: 10.1111/j.1346-8138.1997.tb02522.x. - PubMed
-
- Mazereeuw-Hautier J, Syed S, Leisner RI, et al. Extensive venous/lymphatic malformations causing life-threatening haematological complications. Br J Dermatol 2007; 157: 558-63. doi: 10.1111/j.1365-2133.2007.08003.x. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous