Preclinical immunogenicity and protective efficacy of a SARS-CoV-2 RBD-based vaccine produced with the thermophilic filamentous fungal expression system Thermothelomyces heterothallica C1
- PMID: 37359531
- PMCID: PMC10289020
- DOI: 10.3389/fimmu.2023.1204834
Preclinical immunogenicity and protective efficacy of a SARS-CoV-2 RBD-based vaccine produced with the thermophilic filamentous fungal expression system Thermothelomyces heterothallica C1
Abstract
Introduction: The emergency use of vaccines has been the most efficient way to control the coronavirus disease 19 (COVID-19) pandemic. However, the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern has reduced the efficacy of currently used vaccines. The receptor-binding domain (RBD) of the SARS-CoV-2 spike (S) protein is the main target for virus neutralizing (VN) antibodies.
Methods: A SARS-CoV-2 RBD vaccine candidate was produced in the Thermothelomyces heterothallica (formerly, Myceliophthora thermophila) C1 protein expression system and coupled to a nanoparticle. Immunogenicity and efficacy of this vaccine candidate was tested using the Syrian golden hamster (Mesocricetus auratus) infection model.
Results: One dose of 10-μg RBD vaccine based on SARS-CoV-2 Wuhan strain, coupled to a nanoparticle in combination with aluminum hydroxide as adjuvant, efficiently induced VN antibodies and reduced viral load and lung damage upon SARS-CoV-2 challenge infection. The VN antibodies neutralized SARS-CoV-2 variants of concern: D614G, Alpha, Beta, Gamma, and Delta.
Discussion: Our results support the use of the Thermothelomyces heterothallica C1 protein expression system to produce recombinant vaccines against SARS-CoV-2 and other virus infections to help overcome limitations associated with the use of mammalian expression system.
Keywords: C1; SARS-CoV-2; Thermothelomyces heterothallica; filamentous fungus; hamster; receptor-binding domain; vaccine.
Copyright © 2023 Gonzalez-Hernandez, Kaiser, Steffen, Ciurkiewicz, van Amerongen, Tchelet, Emalfarb, Saloheimo, Wiebe, Vitikainen, Albulescu, Bosch, Baumgärtner, Haagmans and Osterhaus.
Conflict of interest statement
Author GA was employed by company Viroclinics Xplore. Authors RT and ME work for the company Dyadic International, Inc., and may use the vaccine for commercial use. Authors B-JB and BH filed a patent application on coronavirus nanoparticle vaccines. Authors MS, MW, and MV work for the company VTT Technical Research Centre of Finland, Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

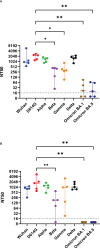


Similar articles
-
Thermophilic Filamentous Fungus C1-Cell-Cloned SARS-CoV-2-Spike-RBD-Subunit-Vaccine Adjuvanted with Aldydrogel®85 Protects K18-hACE2 Mice against Lethal Virus Challenge.Vaccines (Basel). 2022 Dec 11;10(12):2119. doi: 10.3390/vaccines10122119. Vaccines (Basel). 2022. PMID: 36560529 Free PMC article.
-
Toxicity and Local Tolerance of a Novel Spike Protein RBD Vaccine Against SARS-CoV-2, Produced Using the C1 Thermothelomyces Heterothallica Protein Expression Platform.Toxicol Pathol. 2022 Apr;50(3):294-307. doi: 10.1177/01926233221090518. Epub 2022 May 5. Toxicol Pathol. 2022. PMID: 35514116 Free PMC article.
-
A recombinant SARS-CoV-2 receptor-binding domain expressed in an engineered fungal strain of Thermothelomyces heterothallica induces a functional immune response in mice.Vaccine. 2022 Feb 16;40(8):1162-1169. doi: 10.1016/j.vaccine.2022.01.007. Epub 2022 Jan 19. Vaccine. 2022. PMID: 35078661 Free PMC article.
-
Intranasal SARS-CoV-2 RBD decorated nanoparticle vaccine enhances viral clearance in the Syrian hamster model.Microbiol Spectr. 2024 Mar 5;12(3):e0499822. doi: 10.1128/spectrum.04998-22. Epub 2024 Feb 9. Microbiol Spectr. 2024. PMID: 38334387 Free PMC article.
-
An intranasal vaccine comprising SARS-CoV-2 spike receptor-binding domain protein entrapped in mannose-conjugated chitosan nanoparticle provides protection in hamsters.Sci Rep. 2023 Jul 26;13(1):12115. doi: 10.1038/s41598-023-39402-0. Sci Rep. 2023. PMID: 37495639 Free PMC article.
Cited by
-
Rational design of next-generation filovirus vaccines with glycoprotein stabilization, nanoparticle display, and glycan modification.bioRxiv [Preprint]. 2025 Mar 2:2025.03.02.641072. doi: 10.1101/2025.03.02.641072. bioRxiv. 2025. PMID: 40060701 Free PMC article. Preprint.
References
-
- Center for Systems Science and Engineering JHU . COVID-19 dashboard (2021). Available at: https://coronavirus.jhu.edu/map.html.
-
- WHO . Coronavirus disease (COVID-19) pandemic (2022). Available at: https://covid19.who.int/.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

