Efficacy and safety of programmed cell death receptor 1 inhibition-based regimens in patients with pediatric malignancies: the real-world study in China
- PMID: 37359533
- PMCID: PMC10288191
- DOI: 10.3389/fimmu.2023.1182751
Efficacy and safety of programmed cell death receptor 1 inhibition-based regimens in patients with pediatric malignancies: the real-world study in China
Abstract
Background: Programmed death receptor 1 (PD-1) inhibition has shown durable response and mild adverse events (AEs) in adult malignancies. However, data on the clinical activity of PD-1 inhibition in pediatric patients are lacking. We comprehensively assessed the efficacy and safety of PD-1 inhibitor-based regimens for pediatric malignancies.
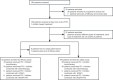
Methods: We conducted a real-world, multi-institutional, retrospective analysis of pediatric malignancies treated with PD-1 inhibitor-based regimens. The primary endpoints were objective response rate (ORR) and progression-free survival (PFS). The secondary endpoints included disease control rate (DCR), duration of response (DOR), and AEs. The Kaplan-Meier method was used to calculate PFS and DOR. The National Cancer Institute Common Toxicity Criteria for AEs (version 5.0) were used to grade toxicity.
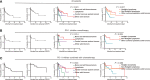
Results: A total of 93 and 109 patients were evaluated for efficacy and safety, respectively. For all efficacy-evaluable patients, PD-1 inhibitor monotherapy, combined chemotherapy, combined histone deacetylase inhibitor, and combined vascular endothelial growth factor receptor tyrosine kinase inhibitor cohorts, the ORR and DCR were 53.76%/81.72%, 56.67%/83.33%, 54.00%/80.00%, 100.00%/100.00%, and 12.50%/75.00%, respectively; the median PFS and DOR were 17.6/31.2 months, not achieved/not achieved, 14.9/31.2 months, 17.6/14.9 months, and 3.7/1.8 months, respectively; the incidence rate of AEs were 83.49%, 55.26%, 100.00%, 80.00%, and 100.00%, respectively. One patient in the PD-1 inhibitor-combined chemotherapy cohort discontinued treatment due to diabetic ketoacidosis.
Conclusions: This largest retrospective analysis demonstrate that PD-1 inhibitor-based regimens are potentially effective and tolerable in pediatric malignancies. Our findings provide references for future clinical trials and practice of PD-1 inhibitors in pediatric cancer patients.
Keywords: PD-1 inhibitor-based treatment; PD-1 inhibitors; efficacy; pediatric malignancies; safety.
Copyright © 2023 Hong, Song, Lan, Wang, Lu, Zhang, Zhu, Sun, Huang, Liu, Xu, Wu, Guo, Cai, Zhen, Que and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.-
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

