Increased plasma level of terminal complement complex in AMD patients: potential functional consequences for RPE cells
- PMID: 37359546
- PMCID: PMC10287163
- DOI: 10.3389/fimmu.2023.1200725
Increased plasma level of terminal complement complex in AMD patients: potential functional consequences for RPE cells
Abstract
Purpose: Polymorphisms in complement genes are risk-associated for age-related macular degeneration (AMD). Functional analysis revealed a common deficiency to control the alternative complement pathway by risk-associated gene polymorphisms. Thus, we investigated the levels of terminal complement complex (TCC) in the plasma of wet AMD patients with defined genotypes and the impact of the complement activation of their plasma on second-messenger signaling, gene expression, and cytokine/chemokine secretion in retinal pigment epithelium (RPE) cells.
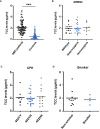
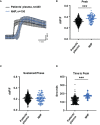
Design: Collection of plasma from patients with wet AMD (n = 87: 62% female and 38% male; median age 77 years) and controls (n = 86: 39% female and 61% male; median age 58 years), grouped for risk factor smoking and genetic risk alleles CFH 402HH and ARMS2 rs3750846, determination of TCC levels in the plasma, in vitro analysis on RPE function during exposure to patients' or control plasma as a complement source.
Methods: Genotyping, measurement of TCC concentrations, ARPE-19 cell culture, Ca2+ imaging, gene expression by qPCR, secretion by multiplex bead analysis of cell culture supernatants.
Main outcome measures: TCC concentration in plasma, intracellular free Ca2+, relative mRNA levels, cytokine secretion.
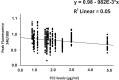
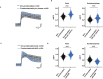
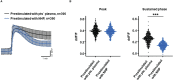
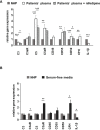
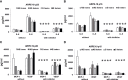
Results: TCC levels in the plasma of AMD patients were five times higher than in non-AMD controls but did not differ in plasma from carriers of the two risk alleles. Complement-evoked Ca2+ elevations in RPE cells differed between patients and controls with a significant correlation between TCC levels and peak amplitudes. Comparing the Ca2+ signals, only between the plasma of smokers and non-smokers, as well as heterozygous (CFH 402YH) and CFH 402HH patients, revealed differences in the late phase. Pre-stimulation with complement patients' plasma led to sensitization for complement reactions by RPE cells. Gene expression for surface molecules protective against TCC and pro-inflammatory cytokines increased after exposure to patients' plasma. Patients' plasma stimulated the secretion of pro-inflammatory cytokines in the RPE.
Conclusion: TCC levels were higher in AMD patients but did not depend on genetic risk factors. The Ca2+ responses to patients' plasma as second-messenger represent a shift of RPE cells to a pro-inflammatory phenotype and protection against TCC. We conclude a substantial role of high TCC plasma levels in AMD pathology.
Keywords: AMD serum; age-related macular degeneration; genetic risk factors; retinal pigment epithelium; terminal complement complex.
Copyright © 2023 Busch, Rau, Sekulic, Perie, Huber, Gehrke, Joussen, Zipfel, Wildner, Skerka and Strauß.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Armstrong RA, Mousavi M. Overview of risk factors for age-related macular degeneration (AMD). J Stem Cells (2015) 10:171–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

