Potency assays and biomarkers for cell-based advanced therapy medicinal products
- PMID: 37359560
- PMCID: PMC10288881
- DOI: 10.3389/fimmu.2023.1186224
Potency assays and biomarkers for cell-based advanced therapy medicinal products
Abstract
Advanced Therapy Medicinal Products (ATMPs) based on somatic cells expanded in vitro, with or without genetic modification, is a rapidly growing area of drug development, even more so following the marketing approval of several such products. ATMPs are produced according to Good Manufacturing Practice (GMP) in authorized laboratories. Potency assays are a fundamental aspect of the quality control of the end cell products and ideally could become useful biomarkers of efficacy in vivo. Here we summarize the state of the art with regard to potency assays used for the assessment of the quality of the major ATMPs used clinic settings. We also review the data available on biomarkers that may substitute more complex functional potency tests and predict the efficacy in vivo of these cell-based drugs.
Keywords: CAR (chimeric antigen receptor); T cell therapy; advanced therapy medicinal product (ATMP); biomarker; potency; stem cell; tissue regeneration.
Copyright © 2023 Capelli, Cuofano, Pavoni, Frigerio, Lisini, Nava, Quaroni, Colombo, Galli, Bezukladova, Panina-Bordignon, Gaipa, Comoli, Cossu, Martino, Biondi, Introna and Golay.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
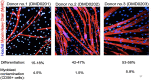
Figures

References
-
- Ramezankhani R, Torabi S, Minaei N, Madani H, Rezaeiani S, Hassani SN, et al. . Two decades of global progress in authorized advanced therapy medicinal products: an emerging revolution in therapeutic strategies. Front Cell Dev Biol (2020) 8:547653. doi: 10.3389/fcell.2020.547653 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

