A Multi-Modal Approach to Islet and Pancreas Transplantation With Calcineurin-Sparing Immunosuppression Maintains Long-Term Insulin Independence in Patients With Type I Diabetes
- PMID: 37359825
- PMCID: PMC10285771
- DOI: 10.3389/ti.2023.11367
A Multi-Modal Approach to Islet and Pancreas Transplantation With Calcineurin-Sparing Immunosuppression Maintains Long-Term Insulin Independence in Patients With Type I Diabetes
Abstract
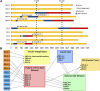
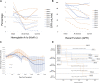
Long-term success in beta-cell replacement remains limited by the toxic effects of calcineurin inhibitors (CNI) on beta-cells and renal function. We report a multi-modal approach including islet and pancreas-after-islet (PAI) transplant utilizing calcineurin-sparing immunosuppression. Ten consecutive non-uremic patients with Type 1 diabetes underwent islet transplant with immunosuppression based on belatacept (BELA; n = 5) or efalizumab (EFA; n = 5). Following islet failure, patients were considered for repeat islet infusion and/or PAI transplant. 70% of patients (four EFA, three BELA) maintained insulin independence at 10 years post-islet transplant, including four patients receiving a single islet infusion and three patients undergoing PAI transplant. 60% remain insulin independent at mean follow-up of 13.3 ± 1.1 years, including one patient 9 years after discontinuing all immunosuppression for adverse events, suggesting operational tolerance. All patients who underwent repeat islet transplant experienced graft failure. Overall, patients demonstrated preserved renal function, with a mild decrease in GFR from 76.5 ± 23.1 mL/min to 50.2 ± 27.1 mL/min (p = 0.192). Patients undergoing PAI showed the greatest degree of renal impairment following initiation of CNI (56% ± 18.7% decrease in GFR). In our series, repeat islet transplant is ineffective at maintaining long-term insulin independence. PAI results in durable insulin independence but is associated with impaired renal function secondary to CNI dependence.
Keywords: immune tolerance; immunosuppression; insulin independence; islet transplant; pancreas transplant.
Copyright © 2023 Wisel, Posselt, Szot, Nunez, Santos-Parker, Gardner, Worner, Roll, Syed, Kelly, Ward, Tavakol, Johnson, Masharani and Stock.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Diabetes C, Complications Trial Research G, Nathan DM, Genuth S, Lachin J, Cleary P, et al. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-dependent Diabetes Mellitus. New Engl J Med (1993) 329(14):977–86. 10.1056/NEJM199309303291401 - DOI - PubMed

