Handling of lipemic samples in the clinical laboratory
- PMID: 37359904
- PMCID: PMC10197190
- DOI: 10.1515/almed-2023-0003
Handling of lipemic samples in the clinical laboratory
Abstract
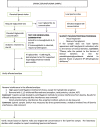
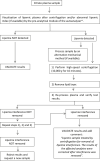
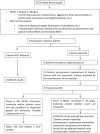
Interferences in the clinical laboratory may lead physicians misinterpret results for some biological analytes. The most common analytical interferences in the clinical laboratory include hemolysis, icterus and lipemia. Lipemia is defined as turbidity in a sample caused by the accumulation of lipoproteins, mainly very-low density lipoproteins (VLDL) and chylomicrons. Several methods are available for the detection of lipemic samples, including the lipemic index, or triglyceride quantification in serum or plasma samples, or mean corpuscular hemoglobin (MCHC) concentration in blood samples. According to the European Directive 98/79/CE, it is the responsibility of clinical laboratories to monitor the presence of interfering substances that may affect the measurement of an analyte. There is an urgent need to standardize interference studies and the way interferences are reported by manufacturers. Several methods are currently available to remove interference from lipemia and enable accurate measurement of biological quantities. The clinical laboratory should establish a protocol for the handling of lipemic samples according to the biological quantity to be tested.
Keywords: interference; intralipid; lipemia; lipemia index; serum indices.
© 2023 the author(s), published by De Gruyter, Berlin/Boston.
Conflict of interest statement
Competing interests: Authors state no conflict of interest.
Figures
References
-
- Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cutpoints-a joint consensus statement from the European atherosclerosis society and European federation of clinical chemistry and laboratory medicine. Clin Chem. 2016;62:930–46. doi: 10.1373/clinchem.2016.258897. - DOI - PubMed
