Electrochemical Impedance Spectroscopy-A Tutorial
- PMID: 37360038
- PMCID: PMC10288619
- DOI: 10.1021/acsmeasuresciau.2c00070
Electrochemical Impedance Spectroscopy-A Tutorial
Erratum in
-
Correction to "Electrochemical Impedance Spectroscopy-A Tutorial".ACS Meas Sci Au. 2025 Jan 31;5(1):156. doi: 10.1021/acsmeasuresciau.5c00007. eCollection 2025 Feb 19. ACS Meas Sci Au. 2025. PMID: 39995601 Free PMC article.
Abstract
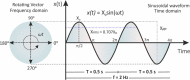
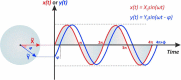
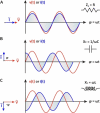
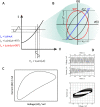
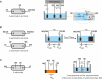
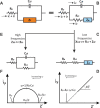
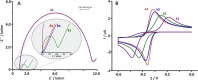
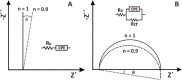
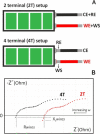
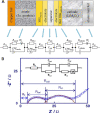
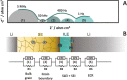
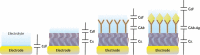
This tutorial provides the theoretical background, the principles, and applications of Electrochemical Impedance Spectroscopy (EIS) in various research and technological sectors. The text has been organized in 17 sections starting with basic knowledge on sinusoidal signals, complex numbers, phasor notation, and transfer functions, continuing with the definition of impedance in electrical circuits, the principles of EIS, the validation of the experimental data, their simulation to equivalent electrical circuits, and ending with practical considerations and selected examples on the utility of EIS to corrosion, energy related applications, and biosensing. A user interactive excel file showing the Nyquist and Bode plots of some model circuits is provided in the Supporting Information. This tutorial aspires to provide the essential background to graduate students working on EIS, as well as to endow the knowledge of senior researchers on various fields where EIS is involved. We also believe that the content of this tutorial will be a useful educational tool for EIS instructors.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
















Similar articles
-
Reducing the resistance for the use of electrochemical impedance spectroscopy analysis in materials chemistry.RSC Adv. 2021 Aug 18;11(45):27925-27936. doi: 10.1039/d1ra03785d. eCollection 2021 Aug 16. RSC Adv. 2021. PMID: 35480766 Free PMC article. Review.
-
Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications.Sensors (Basel). 2021 Oct 1;21(19):6578. doi: 10.3390/s21196578. Sensors (Basel). 2021. PMID: 34640898 Free PMC article. Review.
-
Bioimpedance Spectroscopy: Basics and Applications.ACS Biomater Sci Eng. 2021 Jun 14;7(6):1962-1986. doi: 10.1021/acsbiomaterials.0c01570. Epub 2021 Mar 22. ACS Biomater Sci Eng. 2021. PMID: 33749256 Review.
-
Electrochemical impedance spectroscopy study of corrosion characteristics of palladium-silver dental alloys.J Biomed Mater Res B Appl Biomater. 2021 Nov;109(11):1777-1786. doi: 10.1002/jbm.b.34837. Epub 2021 Apr 4. J Biomed Mater Res B Appl Biomater. 2021. PMID: 33817975
-
Electrochemical Impedance Spectroscopy as a Convenient Tool to Characterize Tethered Bilayer Membranes.Methods Mol Biol. 2022;2402:31-59. doi: 10.1007/978-1-0716-1843-1_4. Methods Mol Biol. 2022. PMID: 34854034
Cited by
-
Regeneration and Long-Term Stability of a Low-Power Eco-Friendly Temperature Sensor Based on a Hydrogel Nanocomposite.Nanomaterials (Basel). 2024 Jan 30;14(3):283. doi: 10.3390/nano14030283. Nanomaterials (Basel). 2024. PMID: 38334553 Free PMC article.
-
Development of dye-sensitized solar cells using pigment extracts produced by Talaromyces atroroseus GH2.Photochem Photobiol Sci. 2024 May;23(5):941-955. doi: 10.1007/s43630-024-00566-x. Epub 2024 Apr 21. Photochem Photobiol Sci. 2024. PMID: 38643418
-
Advanced TiO2-Based Photocatalytic Systems for Water Splitting: Comprehensive Review from Fundamentals to Manufacturing.Molecules. 2025 Feb 28;30(5):1127. doi: 10.3390/molecules30051127. Molecules. 2025. PMID: 40076350 Free PMC article. Review.
-
Luminescent down-shifting layers based on an isoquinoline-Eu(iii) complex for enhanced efficiency of c-Si solar cells under extreme UV radiation conditions.RSC Adv. 2025 Apr 3;15(13):10257-10264. doi: 10.1039/d5ra00584a. eCollection 2025 Mar 28. RSC Adv. 2025. PMID: 40182492 Free PMC article.
-
IrO2-Decorated Titania Nanotubes as Oxygen Evolution Anodes.Molecules. 2025 Jul 10;30(14):2921. doi: 10.3390/molecules30142921. Molecules. 2025. PMID: 40733188 Free PMC article.
References
-
- Barsoukov E.; MacDonald J. R.. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; Wiley-VCH, 2018.
-
- Orazem M. E.; Tribollet B. A Tutorial on Electrochemical Impedance Spectroscopy. ChemTexts 2020, 6 (2), 12.10.1007/s40828-020-0110-7. - DOI
-
- Orazem M.; Tribollet B.. Electrochemical Impedance Spectroscopy, 2nd ed.; Wiley-VCH, 2008.
-
- Bard A. J.; Faulkner L. R.; White H. S.. Electrochemical Methods: Fundamentals and Applications; Wiley-VCH, 2022.
-
- Brett C. M. A.; Brett A. M. O.. Electrochemistry Principles, Methods and Applications; Oxford University Press, 1994. 10.1515/bmte.1999.44.s2.11. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
