Structure of polymeric nanoparticles encapsulating a drug - pamoic acid ion pair by scanning transmission electron microscopy
- PMID: 37360079
- PMCID: PMC10285183
- DOI: 10.1016/j.heliyon.2023.e16959
Structure of polymeric nanoparticles encapsulating a drug - pamoic acid ion pair by scanning transmission electron microscopy
Abstract
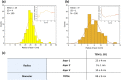
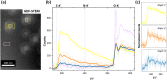
Drug-delivery systems based on polymeric nanoparticles are useful for improving drug bioavailability and/or delivery of the active ingredient for example directly to the cancerous tumour. The physical and chemical characterization of a functionalized nanoparticle system is required to measure drug loading and dispersion but also to understand and model the rate and extent of drug release to help predict performance. Many techniques can be used, however, difficulties related to structure determination and identifying the precise location of the drug fraction make mathematical prediction complex and in many published examples the final conclusions are based on assumptions regarding an expected structure. Cryogenic scanning transmission electron microscopy imaging in combination with electron energy loss spectroscopy techniques are used here to address this issue and provide a multi-modal approach to the characterisation of a self-assembled polymeric nanoparticle system based upon a polylactic acid - polyethylene glycol (PLA-PEG) block copolymer containing a hydrophobic ion-pair between pamoic acid and an active pharmaceutical ingredient (API). Results indicate a regular dispersion of spherical nanoparticles of 88 ± 9 nm diameter. The particles are shown to have a multi-layer structure consisting of a 25 nm radius hydrophobic core of PLA and pamoic acid-API material with additional enrichment of the pamoic acid-API material within the inner core (that can be off-centre), surrounded by a 9 nm dense PLA-PEG layer all with a low-density PEG surface coating of around 10 nm thickness. This structure suggests that release of the API can only occur by diffusion through or degradation of the dense, 9 nm thick PLA-PEG layer either of which is a process consistent with the previously reported steady release kinetics of the API and counter ion from these nanoparticle formulations. Establishing accurate measures of product structure enables a link to performance by providing appropriate physical parameters for future mathematical modelling of barriers controlling API release in these nanoparticle formulations.
Keywords: Controlled release through diffusion barrier; Cryo-STEM; EELS; PLA-PEG; Polymeric nanoparticles.
© 2023 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Natalia Koniuch reports financial support was provided by 10.13039/501100000266Engineering and Physical Sciences Research Council Grant no 2182593.
Figures









Similar articles
-
A novel in situ hydrophobic ion paring (HIP) formulation strategy for clinical product selection of a nanoparticle drug delivery system.J Control Release. 2016 May 10;229:106-119. doi: 10.1016/j.jconrel.2016.03.026. Epub 2016 Mar 18. J Control Release. 2016. PMID: 27001894
-
Comprehensive study of the drug delivery properties of poly(l-lactide)-poly(ethylene glycol) nanoparticles in rats and tumor-bearing mice.J Control Release. 2017 Sep 10;261:31-42. doi: 10.1016/j.jconrel.2017.06.006. Epub 2017 Jun 10. J Control Release. 2017. PMID: 28611009
-
5-Fluorouracil-loaded PLA/PLGA PEG-PPG-PEG polymeric nanoparticles: formulation, in vitro characterization and cell culture studies.Drug Dev Ind Pharm. 2014 Apr;40(4):560-7. doi: 10.3109/03639045.2013.775581. Epub 2013 Apr 18. Drug Dev Ind Pharm. 2014. PMID: 23596973
-
Pegylated poly(lactide) and poly(lactide-co-glycolide) nanoparticles: preparation, properties and possible applications in drug delivery.Curr Drug Deliv. 2004 Oct;1(4):321-33. doi: 10.2174/1567201043334605. Curr Drug Deliv. 2004. PMID: 16305394 Review.
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
Cited by
-
Nanoencapsulation of apocynin and vanillic acid extracted from Picrorhiza kurroa Royle ex Benth plant roots and its characterisation.Heliyon. 2024 Feb 14;10(4):e26156. doi: 10.1016/j.heliyon.2024.e26156. eCollection 2024 Feb 29. Heliyon. 2024. Retraction in: Heliyon. 2025 Feb 18;11(4):142676. doi: 10.1016/j.heliyon.2025.142676. PMID: 38390167 Free PMC article. Retracted.
References
-
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer. 2005;5(3):161–171. - PubMed
-
- Hu Q., et al. Glioma therapy using tumor homing and penetrating peptide-functionalized PEG–PLA nanoparticles loaded with paclitaxel. Biomaterials. 2013;34(22):5640–5650. - PubMed
-
- Labhasetwar V., Song C., Levy R.J. Nanoparticle drug delivery system for restenosis. Adv. Drug Deliv. Rev. 1997;24(1):63–85.
-
- Avgoustakis K. Pegylated poly(lactide) and poly(lactide-co-glycolide) nanoparticles: preparation, properties and possible applications in drug delivery. Curr. Drug Deliv. 2004;1(4):321–333. - PubMed
LinkOut - more resources
Full Text Sources

