Mercuric Chloride Induced Nephrotoxicity: Ameliorative Effect of Carica papaya Leaves Confirmed by Histopathology, Immunohistochemistry, and Gene Expression Studies
- PMID: 37360438
- PMCID: PMC10286259
- DOI: 10.1021/acsomega.3c01045
Mercuric Chloride Induced Nephrotoxicity: Ameliorative Effect of Carica papaya Leaves Confirmed by Histopathology, Immunohistochemistry, and Gene Expression Studies
Retraction in
-
Retraction of "Mercuric Chloride Induced Nephrotoxicity: Ameliorative Effect of Carica papaya Leaves Confirmed by Histopathology, Immunohistochemistry, and Gene Expression Studies".ACS Omega. 2025 Feb 28;10(9):9808. doi: 10.1021/acsomega.5c01751. eCollection 2025 Mar 11. ACS Omega. 2025. PMID: 40092779 Free PMC article.
Abstract
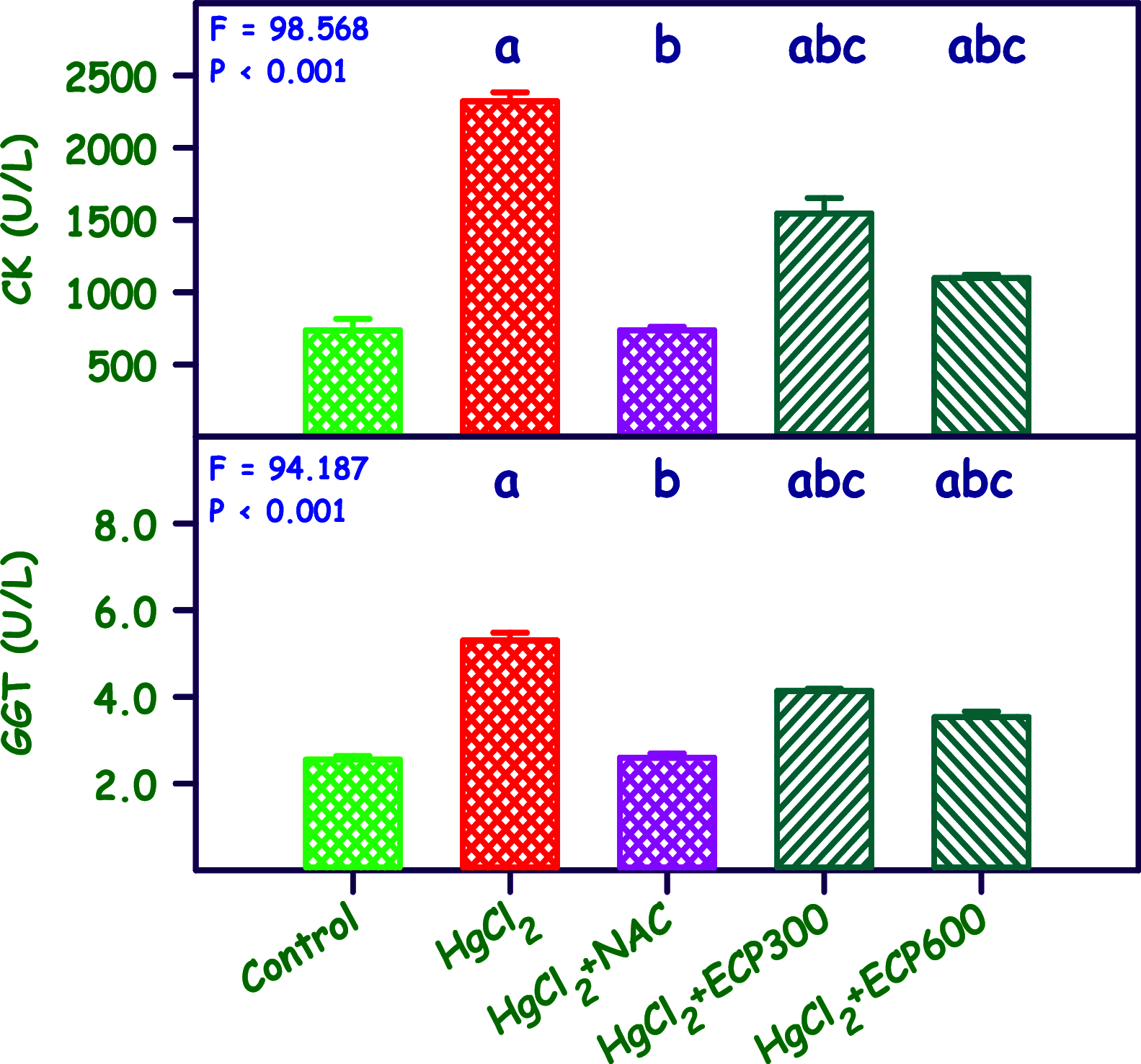
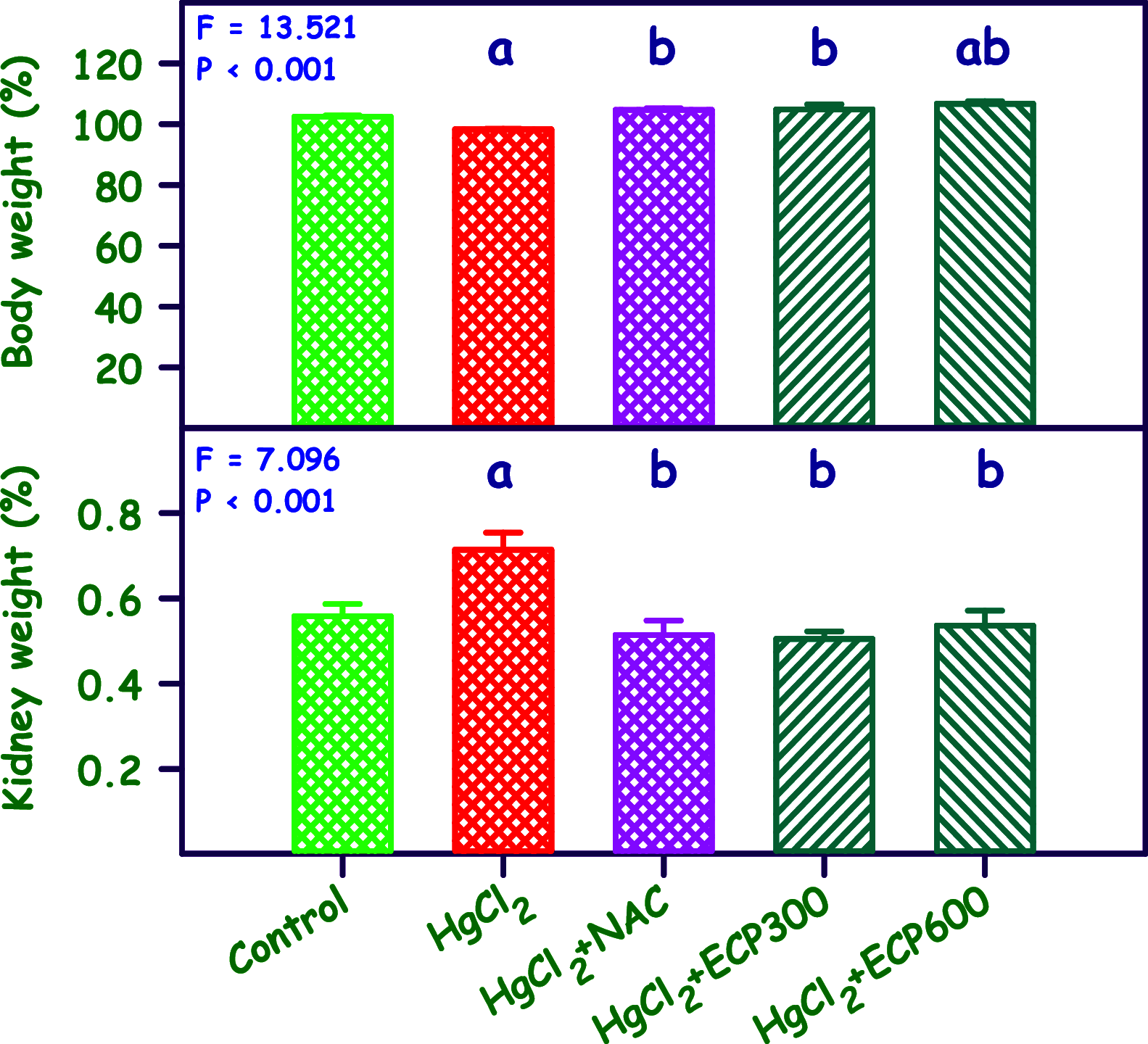
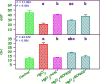
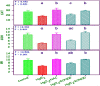
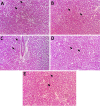
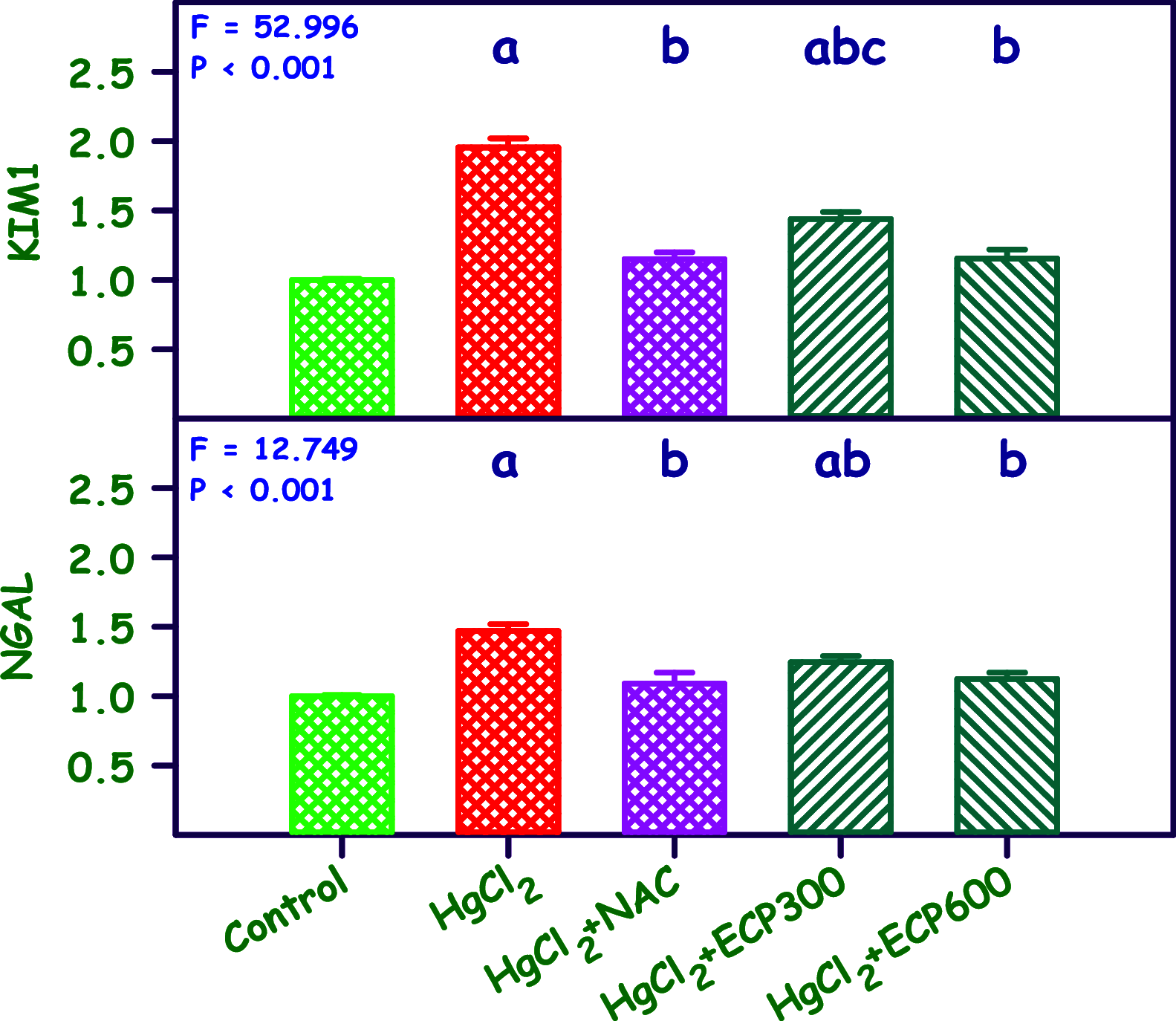
The present study analyzes the efficacy of the ethanolic extract of C. papaya leaves (ECP) against HgCl2-induced nephrotoxicity. The effects on the biochemical and percentage of body and organ weight against HgCl2-induced nephrotoxicity in female Wistar rats were studied. Wistar rats were divided into five groups with six animals in each group: control, HgCl2 (2.5 mg/kg b.w.), N-acetylcysteine (NAC 180 mg/kg) + HgCl2, ECP (300 mg/kg b.w.) + HgCl2, and ECP (600 mg/kg) + HgCl2 groups. After 28 days of study, animals were sacrificed on the 29th day to harvest the blood and kidneys for further analysis. The effect ECP was analyzed by immunohistochemistry (NGAL) and real-time PCR (KIM-1 and NGAL mRNA) in HgCl2-induced nephrotoxicity. The results revealed that the HgCl2 group showed prominent damage in the proximal tubules and glomerulus of nephrons and enormous expression of NGAL in immunohistochemistry and KIM-1 and NGAL in real-time PCR compared to the control group. The simultaneous pretreatment with NAC (180 mg/kg) and ECP (600 and 300 mg/kg) reduced renal damage and expression of NGAL in immunohistochemistry and KIM-1 and NGAL gene in real-time PCR. This study attests to the nephroprotective effect of ECP against HgCl2-induced toxicity.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
In Vivo Antioxidant and Nephroprotective Effects of Ethanolic Extract of Carica papaya Seeds and Its Isolated Flavonoid on Gentamicin-Induced Nephrotoxicity in Wistar Albino Rats.Cureus. 2024 Apr 10;16(4):e57947. doi: 10.7759/cureus.57947. eCollection 2024 Apr. Cureus. 2024. PMID: 38738116 Free PMC article.
-
N-acetylcysteine pretreatment ameliorates mercuric chloride-induced oxidative renal damage in rats.Afr J Med Med Sci. 2010 Dec;39 Suppl:153-60. Afr J Med Med Sci. 2010. PMID: 22416658
-
The Protective Activity of Withania somnifera Against Mercuric Chloride (HgCl2)-Induced Renal Toxicity in Male Rats.Int J Nephrol. 2024 Oct 28;2024:8023989. doi: 10.1155/2024/8023989. eCollection 2024. Int J Nephrol. 2024. PMID: 39502378 Free PMC article.
-
The protective effects of aqueous extract of Carica papaya seeds in paracetamol induced nephrotoxicity in male wistar rats.Afr Health Sci. 2015 Jun;15(2):598-605. doi: 10.4314/ahs.v15i2.37. Afr Health Sci. 2015. PMID: 26124809 Free PMC article.
-
[Experimental study on the effects of BSO, GSH, vitamin C and DMPS on the nephrotoxicity induced by mercury].Wei Sheng Yan Jiu. 2005 Sep;34(5):533-6. Wei Sheng Yan Jiu. 2005. PMID: 16329589 Chinese.
Cited by
-
Therapeutic potential of traditional herbal plants and their polyphenols in alleviation of mercury toxicity.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jul;398(7):7737-7763. doi: 10.1007/s00210-025-03807-7. Epub 2025 Feb 6. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39912903 Review.
-
Thymoquinone modulates oxidative stress and inflammation, correcting mercury-induced HO-1/NRF/Trx pathway disruption in experimental rat hepatorenal system: an in vivo and in silico study.Biometals. 2025 Aug;38(4):1179-1202. doi: 10.1007/s10534-025-00699-1. Epub 2025 Jun 6. Biometals. 2025. PMID: 40481309
-
N-Acetylcysteine Displaces Glutathionyl-Moieties from Hg2+ and MeHg+ to Form More Hydrophobic Complexes at Near-Physiological Conditions.Molecules. 2023 Sep 22;28(19):6762. doi: 10.3390/molecules28196762. Molecules. 2023. PMID: 37836605 Free PMC article.
-
In Vivo Antioxidant and Nephroprotective Effects of Ethanolic Extract of Carica papaya Seeds and Its Isolated Flavonoid on Gentamicin-Induced Nephrotoxicity in Wistar Albino Rats.Cureus. 2024 Apr 10;16(4):e57947. doi: 10.7759/cureus.57947. eCollection 2024 Apr. Cureus. 2024. PMID: 38738116 Free PMC article.
References
-
- Li P.-K.; Garcia G.; Lui S.-F.; Andreoli S.; Fung W.-W.; Hradsky A.; Kumaraswami L.; Liakopoulos V.; Rakhimova Z.; Saadi G.; Strani L.; Ulasi I.; Kalantar Z.-K. Kidney health for everyone everywhere - from prevention to detection and equitable access to care. Clin. Nephrol. 2020, 93, 111–122. 10.5414/CNWKDEditorial. - DOI - PubMed
-
- Bello A.-K.; Levin A.; Tonelli M.; Okpechi I.-G.; Feehally J.; Harris D.; Jindal K.; Salako B. L.; Rateb A.; Osman M. A.; Qarni B.; Saad S.; Lunney M.; Wiebe N.; Ye F.; Johnson D. W. Assessment of Global Kidney Health Care Status. JAMA. 2017, 317, 1864–1881. 10.1001/jama.2017.4046. - DOI - PMC - PubMed
-
- Vaidya V.-S.; Ozer J.-S.; Dieterle F.; Collings F.-B.; Ramirez V.; Troth S.; Muniappa N.; Thudium D.; Gerhold D.; Holder D.-J.; Bobadilla N.-A.; Marrer E.; Perentes E.; Cordier A.; Vonderscher J.; Maurer G.; Goering P.-L.; Sistare F.-D.; Bonventre J.-V. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat. Biotechnol. 2010, 28, 478–485. 10.1038/nbt.1623. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

