Swim in the Chiral Pool: MDMA and MDA Enantiomers from Alanine-Derived Precursors
- PMID: 37360440
- PMCID: PMC10286248
- DOI: 10.1021/acsomega.3c02358
Swim in the Chiral Pool: MDMA and MDA Enantiomers from Alanine-Derived Precursors
Abstract
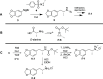
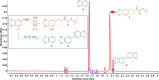
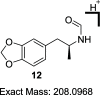
A divergent two-step process has provided access to optically pure enantiomers of MDMA and MDA, clinically relevant phenylisopropylamine entactogens. Target compounds were synthesized from commercially available alanine-derived aziridines. Critical process parameters were identified, and the reactions were optimized to avoid chromatographic purifications toward gram-scale isolations, providing (R)-(-)-MDMA, (S)-(+)-MDMA, (R)-(-)-MDA, and (S)-(+)-MDA each in greater than 98% purity by UPLC, >99% enantiomeric excess, and net yields between 50 and 60% for the complete process.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

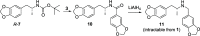
References
-
- Shulgin A. T.History of MDMA. In Ecstasy: The Clinical, Pharmacological and Neurotoxicological Effects of the Drug MDMA. Topics in the Neurosciences; Peroutka S. J., Ed.; Kluwer Academic Publishers: Boston, MA, 1990; vol 9, pp 1–20, 10.1007/978-1-4613-1485-1_1. - DOI
-
- Mitchell J. M.; Bogenschutz M.; Lilienstein A.; Harrison C.; Kleiman S.; Parker-Guilbert K.; Otalora G. M.; Garas W.; Paleos C.; Gorman I.; Nicholas C.; Mithoefer M.; Carlin S.; Poulter B.; Mithoefer A.; Quevedo S.; Wells G.; Klaire S. S.; van der Kolk B.; Tzarfaty K.; Amiaz R.; Worthy R.; Shannon S.; Woolley J. D.; Marta C.; Gelfand Y.; Hapke E.; Amar S.; Wallach Y.; Brown R.; Hamilton S.; Wang J. B.; Coker A.; Matthews R.; De Boer A.; Yazar-Klosinski B.; Emerson A.; Doblin R. MDMA-Assisted Therapy for Severe PTSD: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Study. Nat. Med. 2021, 27, 1025–1033. 10.1038/s41591-021-01336-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

