Functionalized Magnetic Nanoparticles for NIR-Induced Photothermal Therapy of Potential Application in Cervical Cancer
- PMID: 37360441
- PMCID: PMC10286267
- DOI: 10.1021/acsomega.3c01374
Functionalized Magnetic Nanoparticles for NIR-Induced Photothermal Therapy of Potential Application in Cervical Cancer
Abstract
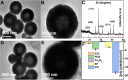
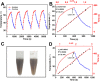
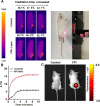
Photothermal therapy (PTT) holds great promise for cancer treatment with its effective ablation of solid tumors. As the essential core point, photothermal agents (PTAs) with excellent photothermal properties and good biocompatibility could help to fulfill highly efficient PTT. Herein, a novel type of nanoplatform Fe3O4@PDA/ICG (FPI) nanoparticle (NP) was designed and synthesized, which was composed of magnetic Fe3O4 and near-infrared excitable indocyanine green via encapsulation of polydopamine. The FPI NPs showed spherical structures in shape with uniform distribution and good chemical stability. Under 793 nm laser irradiation, FPI NPs could generate hyperthermia of 54.1 °C and photothermal conversion efficiency of 35.21%. The low cytotoxicity of FPI NPs was further evaluated and confirmed on HeLa cells with a high survival rate (90%). Moreover, under laser irradiation (793 nm), FPI NPs showed effective photothermal therapeutic characteristics for HeLa cells. Therefore, FPI NPs, as one of the promising PTAs, have great potential in the field of PTT for tumor treatment.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Asami Y.; Yutaka U.; Mamoru K.; Yusuke T.; Sayaka I.; Shinya M.; Eiji K.; Toshitaka M.; Isao M.; Keisuke F.; Yuri I.; Tomio N.; Tadashi K. Epidemiologic and clinical analysis of cervical cancer using data from the population-based Osaka cancer registry. Cancer Res. 2019, 79, 1252–1259. 10.1158/0008-5472.CAN-18-3109. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

