Novel Bioactive Glass/Graphene Oxide-Coated Surgical Sutures for Soft Tissue Regeneration
- PMID: 37360470
- PMCID: PMC10286287
- DOI: 10.1021/acsomega.3c00978
Novel Bioactive Glass/Graphene Oxide-Coated Surgical Sutures for Soft Tissue Regeneration
Abstract
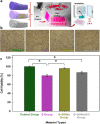
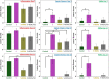
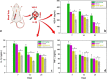
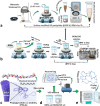
The combination of a commercially available PGLA (poly[glycolide-co-l-lactide]), 90:10% suture material with bioactive bioglass nanopowders (BGNs) and graphene oxide (GO)-doped BGNs offers new opportunities for the clinical application of biomaterials in soft tissue engineering. In the present experimental work, we demonstrate that GO-doped melt-derived BGNs were synthesized via the sol-gel process. After that, novel GO-doped and undoped BGNs were used to coat resorbable PGLA surgical sutures, thereby imparting bioactivity, biocompatibility, and accelerated wound healing properties to the sutures. Stable and homogeneous coatings on the surface of the sutures were achieved using an optimized vacuum sol deposition method. The phase composition, morphology, elemental characteristics, and chemical structure of uncoated and BGNs- and BGNs/GO-coated suture samples were characterized using Fourier transform infrared spectroscopy, field emission scanning electron microscopy, associated with elemental analysis, and knot performance test. In addition, in vitro bioactivity tests, biochemical tests, and in vivo tests were performed to examine the role of BGNs and GO on the biological and histopathological properties of the coated suture samples. The results indicated that the formation of BGNs and GO was enhanced significantly on the suture surface, which allowed for enhanced fibroblast attachment, migration, and proliferation and promoted the secretion of the angiogenic growth factor to speed up wound healing. These results confirmed the biocompatibility of BGNs- and BGNs/GO-coated suture samples and the positive effect of BGNs on the behavior of L929 fibroblast cells and also showed for the first time the possibility that cells can adhere and proliferate on the BGNs/GO-coated suture samples, especially in an in vivo environment. Resorbable surgical sutures with bioactive coatings, such as those prepared herein, can be an attractive biomaterial not only for hard tissue engineering but also for clinical applications in soft tissue engineering.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Development and characterisation of silver-doped bioactive glass-coated sutures for tissue engineering and wound healing applications.Biomaterials. 2004 Mar-Apr;25(7-8):1319-29. doi: 10.1016/j.biomaterials.2003.08.007. Biomaterials. 2004. PMID: 14643606
-
3D-printed photoluminescent bioactive scaffolds with biomimetic elastomeric surface for enhanced bone tissue engineering.Mater Sci Eng C Mater Biol Appl. 2020 Jan;106:110153. doi: 10.1016/j.msec.2019.110153. Epub 2019 Sep 2. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 31753368
-
Bioactive and mechanically strong Bioglass-poly(D,L-lactic acid) composite coatings on surgical sutures.J Biomed Mater Res B Appl Biomater. 2006 Feb;76(2):354-63. doi: 10.1002/jbm.b.30379. J Biomed Mater Res B Appl Biomater. 2006. PMID: 16161126
-
Preparation, Mechanical Properties, and Biocompatibility of Graphene Oxide-Reinforced Chitin Monofilament Absorbable Surgical Sutures.Mar Drugs. 2019 Apr 4;17(4):210. doi: 10.3390/md17040210. Mar Drugs. 2019. PMID: 30987286 Free PMC article.
-
Bioactive glass-based nanocomposites for personalized dental tissue regeneration.Dent Mater J. 2016 Oct 1;35(5):710-720. doi: 10.4012/dmj.2015-428. Epub 2016 Aug 20. Dent Mater J. 2016. PMID: 27546856 Review.
Cited by
-
Development of Curcumin-Loaded TiO2-Reinforced Chitosan Monofilaments for Biocompatible Surgical Sutures.Polymers (Basel). 2025 Feb 12;17(4):484. doi: 10.3390/polym17040484. Polymers (Basel). 2025. PMID: 40006145 Free PMC article.
-
Antibiotic Action, Drug Delivery, Biodegradability, and Wound Regeneration Characteristics of Surgical Sutures and Cutting-Edge Surgical Suture Manufacturing Technologies.J Funct Biomater. 2025 Apr 8;16(4):135. doi: 10.3390/jfb16040135. J Funct Biomater. 2025. PMID: 40278243 Free PMC article. Review.
-
Green Electrospun Poly(vinyl alcohol)/Gelatin-Based Nanofibrous Membrane by Incorporating 45S5 Bioglass Nanoparticles and Urea for Wound Dressing Applications: Characterization and In Vitro and In Vivo Evaluations.ACS Omega. 2024 May 2;9(19):21187-21203. doi: 10.1021/acsomega.4c01102. eCollection 2024 May 14. ACS Omega. 2024. PMID: 38764625 Free PMC article.
-
Sustainable Synthesis of Multifunctionalized Amoxicillin-Loaded Biopolymer Foams.ACS Omega. 2025 Apr 11;10(15):15525-15539. doi: 10.1021/acsomega.5c00442. eCollection 2025 Apr 22. ACS Omega. 2025. PMID: 40290945 Free PMC article.
-
Investigation of Absorbable and Non-Absorbable Multifilament Suture Materials in Terms of Strength Changes Using Chlorhexidine Mouthwash and Thermal Cycling: An In Vitro Study.Materials (Basel). 2024 Aug 4;17(15):3862. doi: 10.3390/ma17153862. Materials (Basel). 2024. PMID: 39124526 Free PMC article.
References
-
- Stamboulis A.; Hench L. L.; Boccaccini A. R. Mechanical Properties of Biodegradable Polymer Sutures Coated With Bioactive Glass. J. Mater. Sci.: Mater. Med. 2002, 13, 843–848. - PubMed
LinkOut - more resources
Full Text Sources

