Immunomagnetic Isolation of HER2-Positive Breast Cancer Cells Using a Microfluidic Device
- PMID: 37360498
- PMCID: PMC10286087
- DOI: 10.1021/acsomega.3c01287
Immunomagnetic Isolation of HER2-Positive Breast Cancer Cells Using a Microfluidic Device
Abstract
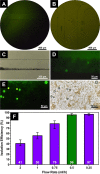
Analysis of circulating tumor cells (CTCs) as a tool for monitoring metastatic cancers, early diagnosis, and evaluation of disease prognosis paves the way toward personalized cancer treatment. Developing an effective, feasible, and low-cost method to facilitate CTC isolation is, therefore, vital. In the present study, we integrated magnetic nanoparticles (MNPs) with microfluidics and used them for the isolation of HER2-positive breast cancer cells. Iron oxide MNPs were synthesized and functionalized with the anti-HER2 antibody. The chemical conjugation was verified by Fourier transform infrared spectroscopy, energy-dispersive X-ray spectroscopy, and dynamic light scattering/zeta potential analysis. The specificity of the functionalized NPs for the separation of HER2-positive from HER2-negative cells was demonstrated in an off-chip test setting. The off-chip isolation efficiency was 59.38%. The efficiency of SK-BR-3 cell isolation using a microfluidic chip with a S-shaped microchannel was considerably enhanced to 96% (a flow rate of 0.5 mL/h) without chip clogging. Besides, the analysis time for the on-chip cell separation was 50% faster. The clear advantages of the present microfluidic system offer a competitive solution in clinical applications.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Two-stage microfluidic chip for selective isolation of circulating tumor cells (CTCs).Biosens Bioelectron. 2015 May 15;67:86-92. doi: 10.1016/j.bios.2014.07.019. Epub 2014 Jul 14. Biosens Bioelectron. 2015. PMID: 25060749
-
Simultaneous on-chip isolation and characterization of circulating tumor cell sub-populations.Biosens Bioelectron. 2020 Nov 15;168:112564. doi: 10.1016/j.bios.2020.112564. Epub 2020 Aug 28. Biosens Bioelectron. 2020. PMID: 32892118
-
Isolation of breast cancer and gastric cancer circulating tumor cells by use of an anti HER2-based microfluidic device.Lab Chip. 2014 Jan 7;14(1):147-56. doi: 10.1039/c3lc51039e. Epub 2013 Nov 7. Lab Chip. 2014. PMID: 24202699 Free PMC article.
-
Recent Advances in Microfluidic Platforms Applied in Cancer Metastasis: Circulating Tumor Cells' (CTCs) Isolation and Tumor-On-A-Chip.Small. 2020 Mar;16(9):e1903899. doi: 10.1002/smll.201903899. Epub 2019 Nov 20. Small. 2020. PMID: 31747120 Review.
-
Microfluidic applications on circulating tumor cell isolation and biomimicking of cancer metastasis.Electrophoresis. 2020 Jun;41(10-11):933-951. doi: 10.1002/elps.201900402. Epub 2020 Mar 17. Electrophoresis. 2020. PMID: 32144938 Review.
Cited by
-
Tumor-on-chip platforms for breast cancer continuum concept modeling.Front Bioeng Biotechnol. 2024 Oct 2;12:1436393. doi: 10.3389/fbioe.2024.1436393. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39416279 Free PMC article. Review.
-
Advances in immunological sorting of X and Y chromosome-bearing sperm: from proteome to sex-specific proteins.Front Vet Sci. 2025 Mar 12;12:1523491. doi: 10.3389/fvets.2025.1523491. eCollection 2025. Front Vet Sci. 2025. PMID: 40144522 Free PMC article. Review.
-
Circulating Tumor Cells as Predictive and Prognostic Biomarkers in Solid Tumors.Cells. 2023 Nov 8;12(22):2590. doi: 10.3390/cells12222590. Cells. 2023. PMID: 37998325 Free PMC article. Review.
-
Doxorubicin-loaded NK exosomes enable cytotoxicity against triple-negative breast cancer spheroids.Iran J Basic Med Sci. 2024;27(12):1604-1615. doi: 10.22038/ijbms.2024.79378.17194. Iran J Basic Med Sci. 2024. PMID: 39539445 Free PMC article.
References
-
- Ruddon R. W.Characteristics of Human Cancer. Cancer Biology; Oxford University Press, 2007; pp 3–15.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

