Improvement of Antioxidant Defences in Keratinocytes Grown in Physioxia: Comparison of 2D and 3D Models
- PMID: 37360501
- PMCID: PMC10290565
- DOI: 10.1155/2023/6829931
Improvement of Antioxidant Defences in Keratinocytes Grown in Physioxia: Comparison of 2D and 3D Models
Abstract
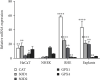
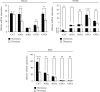
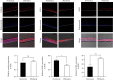
Keratinocytes prevent skin photoaging by ensuring the defence against oxidative stress, an excessive production of reactive oxygen species (ROS). They are localized within the epidermis where the oxygen level (1-3% O2), named physioxia, is low compared to other organs. Oxygen is essential for life but also generates ROS. Most of the in vitro studies on keratinocyte antioxidant capacities are performed under atmospheric oxygen, named normoxia, which is very far from the physiological microenvironment, thus submitting cells to an overoxygenation. The present study is aimed at investigating the antioxidant status of keratinocyte grown under physioxia in both 2D and 3D models. First, we show that the basal antioxidant profiles of keratinocytes display important differences when comparing the HaCaT cell line, primary keratinocytes (NHEK), reconstructed epidermis (RHE), and skin explants. Physioxia was shown to promote a strong proliferation of keratinocytes in monolayers and in RHE, resulting in a thinner epidermis likely due to a slowdown in cell differentiation. Interestingly, cells in physioxia exhibited a lower ROS production upon stress, suggesting a better protection against oxidative stress. To understand this effect, we studied the antioxidant enzymes and reported a lower or equivalent level of mRNA for all enzymes in physioxia conditions compared to normoxia, but a higher activity for catalase and superoxide dismutases, whatever the culture model. The unchanged catalase amount, in NHEK and RHE, suggests an overactivation of the enzyme in physioxia, whereas the higher amount of SOD2 can explain the strong activity. Taken together, our results demonstrate the role of oxygen in the regulation of the antioxidant defences in keratinocytes, topic of particular importance for studying skin aging. Additionally, the present work points out the interest of the choice of both the keratinocyte culture model and the oxygen level to be as close as possible to the in situ skin.
Copyright © 2023 Nadira Chettouh-Hammas et al.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures





Similar articles
-
Human keratinocyte radiosensitivity is linked to redox modulation.J Dermatol Sci. 2006 Jan;41(1):55-65. doi: 10.1016/j.jdermsci.2005.11.008. Epub 2005 Dec 19. J Dermatol Sci. 2006. PMID: 16361084
-
Moringa oleifera stem extract protect skin keratinocytes against oxidative stress injury by enhancement of antioxidant defense systems and activation of PPARα.Biomed Pharmacother. 2018 Nov;107:44-53. doi: 10.1016/j.biopha.2018.07.152. Epub 2018 Aug 2. Biomed Pharmacother. 2018. PMID: 30077837
-
Skin Toxicity Assessment of Silver Nanoparticles in a 3D Epidermal Model Compared to 2D Keratinocytes.Int J Nanomedicine. 2019 Dec 9;14:9707-9719. doi: 10.2147/IJN.S225451. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31849463 Free PMC article.
-
Physiological skin oxygen levels: An important criterion for skin cell functionality and therapeutic approaches.Free Radic Biol Med. 2024 Sep;222:259-274. doi: 10.1016/j.freeradbiomed.2024.06.015. Epub 2024 Jun 20. Free Radic Biol Med. 2024. PMID: 38908804 Review.
-
Impact of reactive oxygen species on keratinocyte signaling pathways.J Dermatol Sci. 2012 Oct;68(1):3-8. doi: 10.1016/j.jdermsci.2012.06.006. Epub 2012 Jun 23. J Dermatol Sci. 2012. PMID: 22771322 Review.
Cited by
-
3D Approaches to Culturing Bovine Skin: Explant Culture versus Organotypic Skin Model.Cells Tissues Organs. 2024;213(5):424-438. doi: 10.1159/000538438. Epub 2024 Mar 21. Cells Tissues Organs. 2024. PMID: 38508156 Free PMC article.
-
Effect of Media Composition and Oxygen Tension on Cellular Stress Response and Nrf2 Activation in HepG2ARE Cells.Antioxidants (Basel). 2025 Jan 24;14(2):137. doi: 10.3390/antiox14020137. Antioxidants (Basel). 2025. PMID: 40002325 Free PMC article.
-
Photodynamically tunable ROS-generating hydrogels for accelerated tissue regeneration.Bioact Mater. 2025 Jul 8;51:977-992. doi: 10.1016/j.bioactmat.2025.05.006. eCollection 2025 Sep. Bioact Mater. 2025. PMID: 40688654 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

