Toward countering muscle and bone loss with spaceflight: GSK3 as a potential target
- PMID: 37360691
- PMCID: PMC10285634
- DOI: 10.1016/j.isci.2023.107047
Toward countering muscle and bone loss with spaceflight: GSK3 as a potential target
Abstract
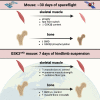
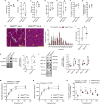
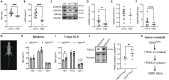
We examined the effects of ∼30 days of spaceflight on glycogen synthase kinase 3 (GSK3) content and inhibitory serine phosphorylation in murine muscle and bone samples from four separate missions (BION-M1, rodent research [RR]1, RR9, and RR18). Spaceflight reduced GSK3β content across all missions, whereas its serine phosphorylation was elevated with RR18 and BION-M1. The reduction in GSK3β was linked to the reduction in type IIA fibers commonly observed with spaceflight as these fibers are particularly enriched with GSK3. We then tested the effects of inhibiting GSK3 before this fiber type shift, and we demonstrate that muscle-specific Gsk3 knockdown increased muscle mass, preserved muscle strength, and promoted the oxidative fiber type with Earth-based hindlimb unloading. In bone, GSK3 activation was enhanced after spaceflight; and strikingly, muscle-specific Gsk3 deletion increased bone mineral density in response to hindlimb unloading. Thus, future studies should test the effects of GSK3 inhibition during spaceflight.
Keywords: Musculoskeletal medicine; Space medicine.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Gillespie J.R., Bush J.R., Bell G.I., Aubrey L.A., Dupuis H., Ferron M., Kream B., DiMattia G., Patel S., Woodgett J.R., et al. GSK-3β function in bone regulates skeletal development, whole-body metabolism, and male life span. Endocrinology. 2013;154:3702–3718. doi: 10.1210/en.2013-1155. - DOI - PMC - PubMed
-
- Kerkela R., Kockeritz L., Macaulay K., Zhou J., Doble B.W., Beahm C., Greytak S., Woulfe K., Trivedi C.M., Woodgett J.R., et al. Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J. Clin. Invest. 2008;118:3609–3618. doi: 10.1172/jci36245. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

