COVID-19 infection among patients with cancer in Australia from 2020 to 2022: a national multicentre cohort study
- PMID: 37360862
- PMCID: PMC10278158
- DOI: 10.1016/j.lanwpc.2023.100824
COVID-19 infection among patients with cancer in Australia from 2020 to 2022: a national multicentre cohort study
Abstract
Background: The global COVID-19 pandemic disproportionately affected certain populations and its management differed between countries. This national study describes characteristics and outcomes of COVID-19 in patients with cancer in Australia.
Methods: We performed a multicentre cohort study of patients with cancer and COVID-19 from March 2020 to April 2022. Data were analysed to determine varying characteristics between cancer types and changes in outcomes over time. Multivariable analysis was performed to determine risk factors associated with oxygen requirement.
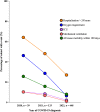
Findings: 620 patients with cancer from 15 hospitals had confirmed COVID-19. There were 314/620 (50.6%) male patients, median age 63.5 years (IQR 50-72) and majority had solid organ tumours (392/620, 63.2%). The rate of COVID-19 vaccination (≥1 dose) was 73.4% (455/620). Time from symptom onset to diagnosis was median 1 day (IQR 0-3), patients with haematological malignancy had a longer duration of test positivity. Over the study period, there was a significant decline in COVID-19 severity. Risk factors associated with oxygen requirement included male sex (OR 2.34, 95% CI 1.30-4.20, p = 0.004), age (OR 1.03, 95% CI 1.01-1.06, p = 0.005); not receiving early outpatient therapy (OR 2.78, 95% CI 1.41-5.50, p = 0.003). Diagnosis during the omicron wave was associated with lower odds of oxygen requirement (OR 0.24, 95% CI 0.13-0.43, p < 0.0001).
Interpretation: Outcomes from COVID-19 in patients with cancer in Australia over the pandemic have improved, potentially related to changing viral strain and outpatient therapies.
Funding: This study was supported by research funding from MSD.
Keywords: COVID-19; Severity; Treatment; Vaccination.
© 2023 The Author(s).
Conflict of interest statement
VGH is supported by an Australian Government National Health and Medical Research Council (NHMRC) post-graduate PhD scholarship (#2014210). BZS has received support from the Fred Hutch Symposium for conference attendance. TT has received honoraria and conference support from Pfizer. CS has sat on advisory boards for MSD, Ipsen, Roche, Astra Zeneca, Sanofi, Janssen and GSK and accepted speaker fees from BMS, Eisai and Novartis. MY has received honorarium from MSD and Takeda. TS has received compensation for serving on scientific advisory boards, honoraria for consultancy and funding for travel from Biogen. MAS has been on data safety monitoring and adjudication committees for Cidara, Roche and Pfizer. She has received research funding from Gilead Sciences, Merck, F2G and sat on advisory boards for Gilead Sciences, F2G, Cidara, Takeda and Merck. MAS is supported by Australian Government National Health and Medical Research Council Investigator (#1173791) and Synergy Grants (#2011100). BWT has been on the advisory board for Moderna, Takeda and CSL-Behring and received research funding from MSD and Seqirus. BWT has received honoraria paid to the institution from Pfizer, Alexion and Janssen. BWT is supported by the Australian Government Medical Research Future Fund Investigator Fellowship (EL-2, #1195894). All other authors declare no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

