Long-term follow-up of patients discontinuing bulevirtide treatment upon long-term HDV-RNA suppression
- PMID: 37360907
- PMCID: PMC10285645
- DOI: 10.1016/j.jhepr.2023.100751
Long-term follow-up of patients discontinuing bulevirtide treatment upon long-term HDV-RNA suppression
Abstract
Background & aims: Bulevirtide (BLV) is a novel antiviral drug licensed for the treatment of chronic hepatitis D. Data on the safety and efficacy of stopping BLV therapy upon long-term HDV-RNA suppression are scarce.
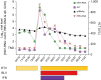
Methods: A total of seven patients (age, 31-68 years, four with cirrhosis) included in a prospective Austrian HDV registry discontinued BLV treatment (duration, 46-141 weeks) upon long-term HDV suppression (HDV-RNA negativity, 12-69 weeks). Pegylated interferon-ɑ2a was used in combination with BLV in two patients. HDV-RNA, alanine aminotransferase, and quantitative HBsAg levels were closely monitored during treatment-free follow-up.
Results: The seven patients were followed up for 14 to 112 weeks. Six patients completed ≥24 weeks of follow-up. HDV-RNA became detectable again in three patients within 24 weeks, whereas one additional patient showed an HDV-RNA relapse after almost 1 year. All patients who relapsed at any point had undergone BLV monotherapy. Meanwhile, HDV-RNA remained undetectable in two patients who were treated with BLV + pegylated interferon-ɑ2a. Only one patient showed significant alanine aminotransferase increases within 24 weeks of follow-up. BLV was reintroduced in three patients after 13-62 BLV-free weeks and was well tolerated, and all patients achieved virologic response again.
Conclusions: BLV discontinuation upon long-term HDV-RNA suppression seems safe. Retreatment with BLV was effective in case of virologic relapse. These findings are within a limited number of patients, and future studies are needed to define stopping rules and further investigate the safety of stopping BLV.
Impact and implications: Limited data exist on stopping bulevirtide (BLV) treatment in patients who achieve long-term HDV-RNA suppression. In a small cohort of seven Austrian patients discontinuing BLV therapy, HDV-RNA relapses were observed in four patients during long-term follow-up, whereas significant alanine aminotransferase increases were recorded in only one. Retreatment with BLV was effective in relapsers. The safety and efficacy of stopping BLV needs to be further studied in larger cohorts.
Keywords: Antivirals; Cirrhosis; Hepatitis D; Treatment; Viral hepatitis.
© 2023 The Authors.
Conflict of interest statement
MJ has served as a speaker for Gilead. PM served as a speaker and/or consultant and/or advisory board member for MSD, AbbVie, Intercept, and Gilead, and received travel support from Gilead and AbbVie. MM served as a speaker and/or consultant and/or advisory board member for AbbVie, Collective Acumen, Gilead, Takeda, and W. L. Gore & Associates, and received travel support from AbbVie and Gilead. MT served as a speaker and/or consultant and/or advisory board member for Abbvie, Albireo, BiomX, Boehringer Ingelheim, Bristol-Myers Squibb, Falk, Genfit, Gilead, Hightide, Intercept, Janssen, MSD, Novartis, Phenex, Regulus, Siemens, and Shire, and received travel support from AbbVie, Falk, Gilead, Intercept, and Jannsen, as well as grants/research support from Albireo, Alnylam, Cymabay, Falk, Gilead, Intercept, MSD, Takeda, and Ultragenyx. Moreover, he is a co-inventor of patents on the medical use of 24-norursodeoxycholic acid. HZ received speaker honoria from the Abbvie, Bayer, BMS, Falk Foundation, Gilead, Intercept, Merck, MSD, Novartis, Pierre-Fabre, Pharmacosmos, and Vifor; he has advised for Abbvie, Bayer, Eisai, Gilead, Intercept, MSD, Novartis, Novo Nordisk, Shire, Pierre-Fabre, Pharmacosmos, and Vifor. He further received travel grants from Abbvie, Bayer, Gilead, and Intercept, and research grants from Abbvie, Gilead, MSD, Novartis, Pharmacosmos, and Vifor. TR served as a speaker and/or consultant and/or advisory board member for AbbVie, Bayer, Boehringer Ingelheim, Gilead, Intercept, MSD, Siemens, and W. L. Gore & Associates, and received grants/research support from AbbVie, Boehringer Ingelheim, Gilead, MSD, Philips, and W. L. Gore & Associates, as well as travel support from Boehringer Ingelheim and Gilead. PF received an unrestricted research grant from Gilead, was a member of the safety review Committee for MyrPharma, received speaking honoraria from Gilead and Abbvie, and received a consulting/advisory board fee from Vivaraxx. MP, LH, MSch, and LB have nothing to disclose. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



References
-
- European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. - PubMed
-
- Wedemeyer H., Yurdaydìn C., Dalekos G.N., Erhardt A., Çakaloğlu Y., Değertekin H., et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med. 2011;364:322–331. - PubMed
-
- Wedemeyer H., Yurdaydin C., Hardtke S., Caruntu F.A., Curescu M.G., Yalcin K., et al. Peginterferon alfa-2a plus tenofovir disoproxil fumarate for hepatitis D (HIDIT-II): a randomised, placebo controlled, phase 2 trial. Lancet Infect Dis. 2019;19:275–286. - PubMed
LinkOut - more resources
Full Text Sources

